Study
Treatment arm
Control arm
Number of patients
Outcomes
Treatment arm vs control arm (%)
p-value
Takayama et al. [9]
CIK
Radical resection
150
5-year DFS
38 vs 22
p < 0.05
Radical resection
5-year OS
68 vs 62
p>0.05
Weng et al. [11]
CIK
TACE + RFA
85
1-year recurrence
9 vs 30
p < 0.05
TACE + RFA
1.5-year OS
100 vs 100
p>0.05
Hui et al. [10]
CIK + IL-2
Radical resection
127
5-year DFS
23 vs 11
p < 0.05
Radical resection
5-year OS
38 vs 37
p > 0.05
Kawata et al. [8]
LAK + IL-2
adriamycin
24
3-year DFS
50 vs 25
p>0.05
Adriamycin
3-year OS
71 vs 74
p>0.05
Clinical trials of immunotherapy using other cells than CIK or LAK are ongoing or were completed according to the databases of clinical trials. According to ClinicalTrial.gov, eight cell-based immunotherapies were conducted as clinical trials so far (Table 5.2). Innate lymphoid cells including NK cells, NKT cells and γδT cells were used as effector cells in 3 trials. No evidence was demonstrated by phase III clinical trials with those innate lymphoid cells so far in any types of cancer, but in theory NK cells, NKT cells or γδT cells can target lack of MHC class I on cancer cells or phosphate antigen induced by abnormal metabolism in the tumor microenvironment. Tumor-infiltrating lymphocytes in 2 trials or dendritic cells in 2 trials were used to target cancers by tumor-specific immune responses.
Table 5.2
Clinical trials of immunotherapy for HCC registered in ClinicalTrial.gov
Cell type | Cancer | Phase | Facility | ID |
|---|---|---|---|---|
γδT | HCC | I | Rennes University Hospital | NCT00562666 |
NK, IL-2-activated | HCC | I | University of Miami | NCT01147380 |
NK & NKT | Various cancers including HCC | I | Envita Medical Center, Inc. | NCT00909558 |
TIL | HCC, nasopharyngeal, breast | I | Sun Yat-sen University | NCT01462903 |
DC loaded with AFP peptides | HCC | I/II | Nantes University Hospital | NCT01128803 |
DC | HCC, melanoma, renal cell | II | Clinica Universidad de Navarra | NCT00610389 |
CD8+ TIL | Metastatic cancers including HCC | II | National Cancer Institute, NIH | NCT01174121 |
CIK | HCC | III | Sun Yat-sen University | NCT01749865 |
In Japan, seven cell-based immunotherapies were conducted as clinical trials so far according to UMIN (Table 5.3). Two trials were γδT cell-based immunotherapy. Four trials were DC-based immunotherapy which intended to induce HCC-specific immune responses. As tumor antigen, tumor lysate was pulsed in DC in 1 trial (UMIN000005820). To load more specific tumor antigen in DC, tumor antigen mRNA-encoding DC was used in 1 trial (UMIN000005836). DC stimulated with OK-432, inactivated streptococcus pyogenes, were used in 1 trial to activate DC (UMIN000001701). Thus, various cell types and techniques were examined in the current clinical trials to enhance anti-tumor immune responses against HCC.
Table 5.3
Clinical trials of immunotherapy for HCC registered on UMIN in Japan
Cell type | Cancer | Phase | Facility | UMIN ID |
|---|---|---|---|---|
Naïve T cells | HCC | II | Kyoto Prefectural University of Medicine | UMIN000003861 |
γδT | HCC | pilot | Tokyo Medical University | UMIN000004583 |
γδT | HCC | pilot | The University of Tokyo | UMIN000001418 |
DC | HCC | pilot | Tokyo Medical University/The University of Tokyo | UMIN000000971 |
DC, encoding cancer antigen mRNA | HCC, pancreatic | pilot | Yamaguchi University | UMIN000005836 |
DC, OK432-stimulated | HCC | I/II | Kanazawa University | UMIN000001701 |
DC, tumor lysate-pulsed | Various cancers including HCC | I/II | Tokyo Women’s Medical University | UMIN000005820 |
5.3 DC-Based Immunotherapy for HCC
Among various types of cell-based immunotherapy, sipuleucel-T is the only therapy which was verified to prolong overall survival of prostate cancer in phase III trial [7]. Sipuleucel-T comprises DC pulsed with tumor-specific antigen, PAP. If tumor-antigen is known, DC-based immunotherapy is a promising therapy to treat HCC according to the success in sipuleucel-T. Palmer reported that phase II trial of tumor lysate-pulsed DC infusion for patients with unresectable advanced HCC achieved disease control rate 28 % (combined partial response and stable disease) [15]. In the trial, hepatocellular carcinoma cell line HepG2 was used for source of tumor lysate. What is the best to load in DC, autologous tumor lysate, cell line-derived lysate, or HCC-specific peptides, is still controversial and to be determined in future.
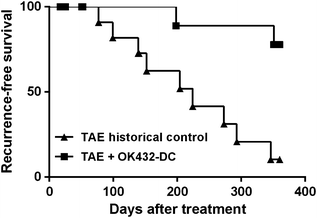
We activated autologous DC from cancer patients with OK-432 before the infusion [16]. OK432-activated DC expressed high level of co-stimulatory molecules like CD80, CD83, and CD86. OK-432-stimulated DC also highly produced IL-12p40 and IFN-γ and revealed high tumoricidal activity against HCC cell lines like Hep3B and PLC/PRF/5 in in vitro assay. After TAE OK-432-stimulated DC were injected in hepatic artery via catheter. OK432-stimulated DC significantly prolonged recurrence-free survival compared to historical TAE control (P = 0.0017; Fig. 5.1). Although IFN-γ responses with PBMC specific to hTERT which is highly expressed by HCC as tumor antigen didn’t significantly increase after the OK-432-stimulated DC treatment, serum level of TNF, IL-9 and IL-15 was significantly higher in OK-DC group than TAE control patients. In mouse model of OK-DC treatment for HCC, OK-DC clearly induced tumor-specific immune responses against colon cancer cells [17]. It is still controversial that under which mechanism OK-432-stimulated DC exerts anti-tumor effect, tumor-specific immunity or non-specific direct killing. Although the mechanisms underlying the prolonged DFS caused by OK-432-stimulated DC treatment should still be elucidated, DC-based immunotherapy is a promising treatment for HCC.