Disease
Reference
Nonalcoholic steatohepatitis
[21]
Hepatitis
Fulminant hepatitis
Liver injury
Liver fibrosis
[11]
Rejection after the transplantation
[26]
Obesity
Diabetes
Alpha-1 antitrypsin deficiency
[12]
Table 8.2
The establishment of the animal disease model using hydrodynamic delivery
Disease model | Genes and cells delivered | Reference |
|---|---|---|
Hypertriglyceridemia | Lipoprotein lipase | [30] |
Hepatitis B | Viral genome | [31] |
Hepatitis C | Viral genome | [32] |
Hepatitis D | Viral genome | [33] |
Liver fibrosis | Transforming growth factor-beta1 | [34] |
Liver cancers | c-met | [35] |
Multiorgan metastasis | B16-F1 melanoma cells | [36] |
4 T1 breast cancer cells | [36] | |
Renca renal carcinoma cells | [36] |
8.2.2 Image-Guided Hydrodynamic Delivery
The image-guided catheter insertion technique has been employed to achieve liver-directed and lobe-specific hydrodynamic delivery in large animals [15]. The image-guided hydrodynamic gene delivery is performed in a site-specific manner based on the site of catheter insertion in liver. Kamimura et al. reported a procedure in 2009 in pigs [15], and similar studies were also reported by others [13, 14, 19]. At experimental level, a skin incision is made at the neck to expose the jugular vein and to insert a peripheral i.v. catheter. A guide wire is then inserted through the peripheral catheter and later replaced with a short introducer. The guide wire is inserted into the selected hepatic vein, e.g., the right lateral hepatic vein in the right lateral lobe, under X-ray image guidance. An injection catheter with a balloon on its tip is then inserted through the introducer into the targeted liver lobe following the guide wire. Inflation of the balloon is achieved by injecting a small volume of phase contrast medium to block blood flow. The blockade is verified by injecting a phase contrast medium into the vasculature through the injection catheter (Fig. 8.1a). Hydrodynamic injection is then performed directly into the targeted hepatic lobe (Fig. 8.1a). The efficiency of this procedure was demonstrated in pig livers [15, 17]. Because the injection is localized and specifically to a given area in the liver, the injection volume is significantly reduced from 10 % BW in volume of a mouse to 1.25 % BW in pigs [15, 17]. Target-specific gene delivery was confirmed at the area near the injection site (Fig. 8.1b). Gene delivery efficiency, however, is dependent on the position of the catheter insertion in the selected liver lobe, intravascular pressure, and injection volume [15, 17]. Efforts have been made to optimize these parameters for optimal efficiency and safety of the procedure in gene delivery to pig livers and skeleton muscles [16, 17]. The optimum condition achieved so far is capable of achieving expression of the human alpha-1 antitrypsin gene in livers for more than 2 months after gene delivery [17].
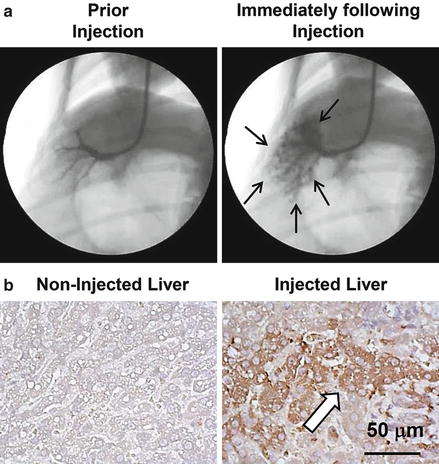

Fig. 8.1
Image-guided, computer-assisted hydrodynamic gene delivery. (a) Location of the balloon catheter in the hepatic vein of dog liver. Black arrows represent the distribution of the contrast medium in the injected liver lobe. (b) Immunohistochemical staining of the liver. Liver samples from the control and plasmid DNA-injected dog liver were stained with anti-luciferase antibodies. Scale bar represents 50 μm. White arrow indicates hepatocytes expressing transgene (taken from Kamimura et al. [18] with permission)
To further extend the clinical applicability of this method, the safety of the procedure was also evaluated in dogs [18]. Histological examination and hepatic microcirculation measurements using reflectance spectrophotometry revealed a transient expansion of the liver sinusoids in the injected area with no change in other areas or organs. Serum biochemistry also showed a transient increase in concentrations of liver enzymes and cytokines related to vascular stretching upon injection. Physiological parameters including electrocardiogram, heart rate, blood pressure, oxygen saturation, and body temperature remained normal during and after hydrodynamic injection, and no changes in BW were observed in dogs that underwent three hydrodynamic injections in 6 weeks at 2-week time intervals. Importantly, no transfer of the injected plasmid was observed in other organs. These results suggest that the image-guided hydrodynamic delivery procedure is site specific, safe, and effective for long-term gene expression.
8.2.3 Development of Clinically Applicable Injection Device
The first generation of the image-guided hydrodynamic gene delivery system was developed in 2007 by Suda et al. [37] using the pressure of a CO2 tank to drive the injection. Kamimura et al. have recently reported a different system utilizing an electric power-driven motor as a driving force, instead of pressurized gas [38]. The design of the new system has desirable computerized intravascular pressure profile to guide the injection [38] (Fig. 8.2). Once the injection starts, the electric motor pumps the DNA solution into the target organ and consequently increases the vascular pressure at the tip of inserted catheter. Based on the pressure profile preloaded in the computer, the injection can be self-adjusted based on an algorithm in the computer. The major advantage of the motor-driven system is that it can eliminate the possibility of gas embolism that could occur when a CO2 tank is used to drive the injection. Current efforts are to evaluate the efficacy of the new system for treatment of a various diseases, in an animal model [39], and to develop a computer program with optimized injection parameters for a safe and efficient gene delivery under any given defined condition. Clinical trials would be the next logic step bridging the image-guided, computer-assisted hydrodynamic gene delivery to clinical application on human patients.
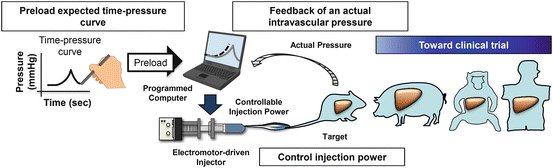

Fig. 8.2
Computer-assisted hydrodynamic gene delivery system (modified from Yokoo et al. [38] with permission). (1) Various time–pressure curves are preloaded to a computer. (2) The electric power-driven injector starts hydrodynamic injection to animals. (3) The actual intravascular pressure in the target organ is transmitted to the computer instantly. (4) Computer self-adjusts motor-driven injection to match the preloaded pressure profiles. Cartoon animals represent the animal species that hydrodynamics-based procedure has been successfully performed toward clinical trial in humans
8.3 Conclusion
Hydrodynamic delivery was originally established for gene therapy studies in mice. It has advanced in the past 15 years to a stage where this technique can be employed to achieve site-specific, safe, and effective gene delivery in combination with other technologies such as imaging, intravascular catheterization, and computer-assisted regulation. It is highly possible that this new technology will proceed beyond nucleic acid delivery into delivery of other pharmaceutics for treatment of diseases that conventional therapeutic methods are insufficient or unavailable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







