Primary tumor
Incidence (%)
Disease-specific considerations in PSM
Reference
CRC
30
Cause of disease-specific mortality in 30 %
Esquivel et al. [40]
Small bowel
40
Synchronous PSM at time of diagnosis in 50 %
Brücher et al. [6]
Stomach (pT3/4)
50
Synchronous PSM in 50 % at time of first surgical exploration
Xu et al. [7]
Ovarian
75
Synchronous PSM at time of diagnosis in 50 %
Armstrong et al. [8]
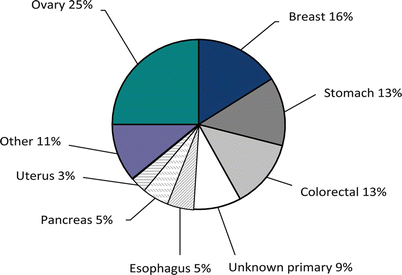
Peritoneal surface malignancy of CRC origin is a frequent manifestation in the natural history of the disease, and it is associated with marked deterioration in quality of life (QOL) and very poor prognosis. Peritoneal disease spread continues to be a common mode of disease progression for intra-abdominal malignancies. Eight percent of patients with CRC have synchronous peritoneal spread of disease at time of primary resection, and up to 25 % of patients with recurrent CRC have disease confined to the peritoneal cavity [3]. In about 30 % of patients with CRC, PSM is the main reason for disease-specific mortality [9]. On the positive side, ~50 % of patients who develop PSM from CRC may have curative treatment by an R0-resection.
Confinement of disease to a limited extent of the peritoneal surface in the absence of systemic spread of disease has served as the basis for surgical eradication of disease through aggressive CRS + HIPEC. Survival during the time when patients with PSM from CRC had been treated by systemic therapy alone, typically 5-FU, was limited to approximately 6 months (range 5–7 months) [9, 10]. Among patients who suffered from PSM due to CRC and had simultaneous malignant bowel obstruction, the survival was even worse—limited to 3 months [11]. Based on recent experience, however, a paradigm shift has occurred.
Change of Paradigm
Key Concept: The results that can be obtained with cytoreduction and heated intraperitoneal chemotherapy for resectable peritoneal surface malignancy of colorectal cancer origin are similar to hepatic resection for resectable colorectal cancer metastasis, with 5–year overall survival of ~45 %.
A clear change of paradigm occurred slowly within the past 50 years, in part due to the increasing recognition that PSM is a regional disease once limited to a compartment—the abdomen. It was not, as once thought, a systemic disease for which only palliative intervention was indicated [3]. It was not until the 1980s that the generally held fatalistic view of PC gave way to a new way of thinking with regard to treatment options and treatment-specific prognosis; such options expanded beyond purely palliative and/or best supportive therapy. During the 1990s, pioneering surgeons such as Paul Sugarbaker and Francois Gilly were the principal driving forces that moved away from that fatalistic approach toward a curative treatment approach by using CRC + HIPEC in carefully selected patients that could benefit from such an aggressive treatment intervention [3, 4, 12–16]. Disease once limited to bleak outcomes of 3–6 month median survival with therapy could, in selected cases, be treated aggressively with CRS + HIPEC and have strikingly improved outcomes [9, 10]. In fact, patients undergoing complete resection of PSM from CRC followed by HIPEC could attain median survival of 21–40 months, while patients with pseudomyxoma peritonei were reported to have 20-year survival of up to 70 % [17]. However, these results clearly depended on the extent of peritoneal surface tumor burden and completeness of cytoreduction [18]. The curative treatment approach in PC is a demanding and complex interdisciplinary procedure in which surgeons, anesthetists, oncologists, gastroenterologists, dieticians, physical and occupational therapists, psychologists, and case managers, among others, should be equally involved in the patient-centered, integrative, team approach to cancer care. It must be emphasized that CRS, HIPEC, and systemic therapy are not competitive therapies, and this can be recognized by the fact that in France this therapeutic paradigm has already incorporated into French Guidelines for standards of practice [19]. In 2012, Germany integrated this approach into national treatment guidelines as a therapeutic option [20]. Surgical oncologists caring for patients with PC need a wide range of training and experience that extends well beyond the technical aspects of surgical care and includes understanding of the biology of disease, assessment of the extent of disease, careful patient selection, administration of HIPEC, and related anesthetic and safety considerations, as well as postoperative interventions for secondary surgical events. Understanding of the fundamentals of peritoneal surface disease-specific anatomy and embryology is essential.
Anatomy and Embryology
Key Concept: The pelvic–peritoneal partition serves as the anatomic basis for the delivery of dose–dense heated intraperitoneal chemotherapy.
A detailed description of the ultrastructure of the peritoneum was published by Baron in 1941 [21] and reviewed recently by us [20]. The distinct histological structure of the peritoneum is evident in a special type of vascular anatomy and also its specific function. The peritoneum consists of a single-cell layer of mesothelial cells, with a basal membrane beneath it along with five layers of connective tissue (interstitial cells and a matrix of collagen, hyaline, and proteoglycans), with a total thickness of 90 μm [3, 22]. As it also contains other cellular elements such as pericytes, parenchymal cells, and blood capillary vessels, the peritoneum is often referred to as the “peritoneal membrane.” The functions of the peritoneum include maintenance of the mobility of intra-abdominal organs relative to the abdominal wall. This is achieved through a lubricant secreted by the peritoneal membrane consisting of glycosaminoglycans and phospholipids. The membrane further fulfills an important function in defense against intra-abdominal infections. It is also thought that the peritoneum represents the principal barrier and initial line of defense against dissemination of malignant cells and establishment of peritoneal carcinomatosis [23]. This view is supported by research, which has shown that intraperitoneal injection of aggressive tumor cell lines leads to a corresponding increase in tumor cell activity in the peritoneal membrane [3]. The interaction between its single layer of mesothelial cells together with associated blood capillaries and surrounding interstitial matrix contributes to this line of defense [24]. In fact, the peritoneal membrane is regarded as an organ itself [3] and its surface area approximates 7,500 cm2 and is in direct contact with all intra-abdominal organs.
At the end of the third week of gestation, the intraembryonic mesoderm divides bilaterally into the mesoderm, the intermediate mesoderm, and the lateral plate. In the lateral plate, a mesothelial cell layer divides into the parietal and visceral mesoderm. The parietal mesoderm, which lines the intraembryonic celomic cavity, becomes the parietal peritoneum, the parietal pleura, and the pericardium. From the visceral mesodermal layer, the visceral peritoneum, visceral pleura, and epicardium develop. The dorsal mesentery, to which the intestinal tube is attached, represents the junction between the parietal and visceral peritoneum. Understanding this embryology and anatomical relationship is important in the technical execution of cytoreduction [3]. It is also important to recognize that there is practically never any tumor penetration into the underlying organ structures (e.g., kidney, spleen) in cases of PC. This is probably due to the peritoneum’s embryologically delineated barrier function.
Classification and Types of Growth of PC
Key Concept: Irrespective of the growth pattern of peritoneal surface malignancy, the predominant factor–determining outcome is the ability to achieve complete cytoreduction.
Peritoneal surface malignancy can be subdivided into primary and secondary forms [3]. Primary PSM consists of invasion by a mesothelioma or pseudomyxoma peritonei—both extremely rare tumor entities. Secondary PC originates most commonly from gastrointestinal tumors [25–27] or urogenital tumors [28]. Other forms of secondary PC involve less common primary epithelial malignancies such as malignant melanoma or breast carcinoma. There are important differences between growth types in peritoneal carcinomatosis pertaining to involvement of the bowel, supporting mesentery, and its critical vascular structures; these are important to consider when estimating likelihood of achieving complete cytoreduction with CRS, particularly when there is substantial involvement of the mesenteric pedicle, or root of the mesentery (Fig. 5.1), [3].
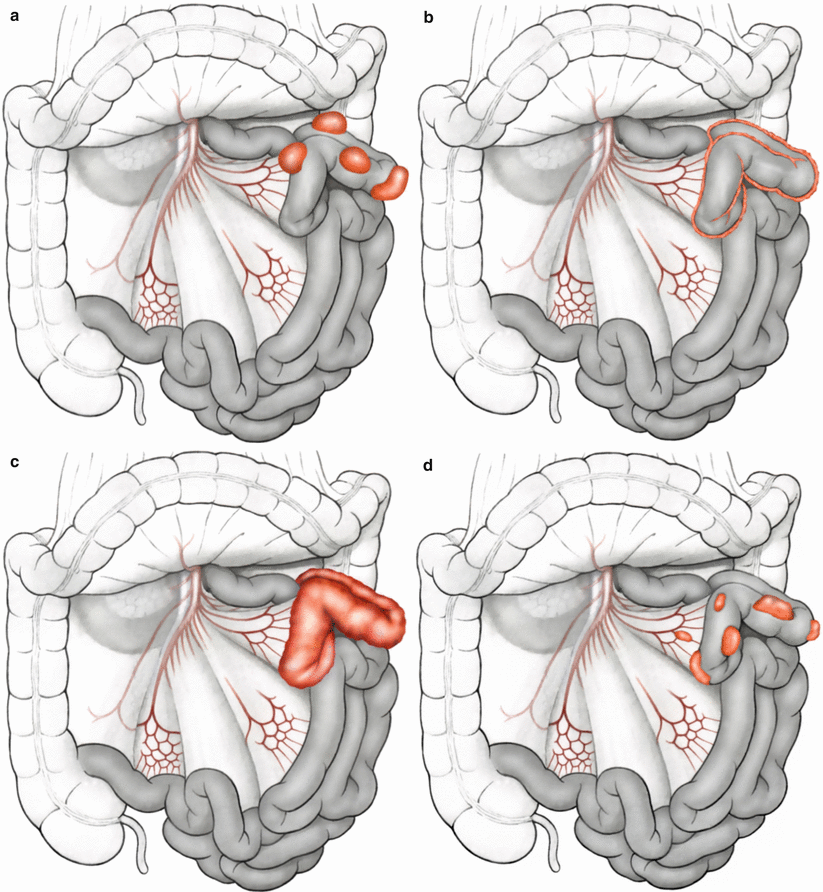
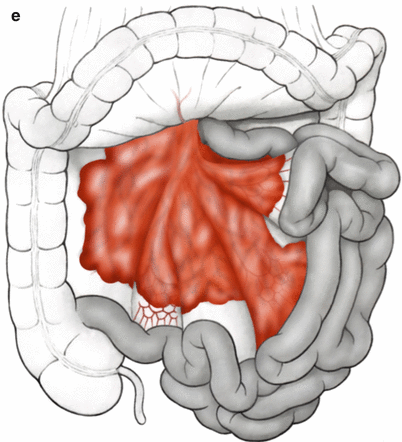


Fig. 5.1
(a–e) Growth patterns in peritoneal carcinomatosis on the small bowel (Modified from Brücher et al. [3])
History and Rationale for Intraperitoneal Drug Therapy
Key Concept: Heated intraperitoneal chemotherapy is indicated for treatment of non–visible or <1 mm peritoneal surface tumor deposits.
The history of intraperitoneal drug therapy was reported recently [20]. The earliest report mentioned in the literature about the use of intraperitoneal “drug therapy” was by the English surgeon, Christopher Warrick in 1744 [29]. The Belgium surgeon, WP Ceelen, together with a US colleague, MF Flessner, reported on the biophysics of intraperitoneal therapy [30] that Warrick injected into the peritoneal cavity, a mixture of “Bristol” water and “claret,” a Bordeaux wine, in the female, Jane Roman, who suffered from malignant ascites. The cytotoxic nitrogen mustard, which had been in use during World War II, was investigated in the 1950s in clinical trials for the purpose of intraperitoneal therapy [31]. In 1978, Dedrick reported about the pharmacokinetics of intraperitoneal drug delivery, distribution, and clearance given the peritoneal–plasma partition. This anatomical barrier provides the fundamental rationale for intraperitoneal drug delivery, such that a much higher drug concentration can be used than administered systemically, because peritoneal drug clearance is much slower than plasma clearance [32]. Intraperitoneal drug delivery has been proven to be efficient and effective in patients with minimal (“infra-millimetric”) or microscopic residual disease following cytoreductive surgery [30]. Hence, cytoreductive surgery is intended to clear visible peritoneal surface disease, while HIPEC is indicated for treatment of non-visible or <1 mm peritoneal tumor deposits, as intraperitoneal chemotherapy penetrates only a millimeter in depth during HIPEC. The reason why intraperitoneal therapy emerged early in the history of regional therapy seems to be related to the challenge of alleviating symptomatic malignant ascites. There are various epithelial malignancies that may lead to symptomatic ascites; these are shown in Fig. 5.2 [33]. Malignant ascites reflects a symptom of peritoneal carcinomatosis, and it indicates the presence of malignant cells within the peritoneal cavity. The biodynamic effects of intraperitoneal drug administration were shown to be dependent on a number of key variables, such as diffusion and convection (dependent on molecular weight of the agent administered), and interstitial fluid pressure; malignant tumors characteristically have elevated interstitial fluid pressure, which serves as a barrier for connective drug transport. Flessner et al. showed that the structure of the peritoneal intracellular matrix is the major source of resistance to macromolecular drug transport [34]. The tumor penetration distance measured experimentally ranges from a few cell layers (generally <1 mm) to a maximum of 3–5 mm [30]. Active and passive transport across the cell membrane leads to better and somewhat worse intracellular drug concentration, and the mode of transport influences the efficacy of regional drug application. Additionally, in the case of cisplatin, the copper transport protein-1(CTR1) regulates uptake in human cancer cells [35]. Additionally, preclinical models have shown that hypotonic carrier fluids lower interstitial fluid pressure and increase intraperitoneal pressure, leading to enhanced peritoneal drug penetration [30].
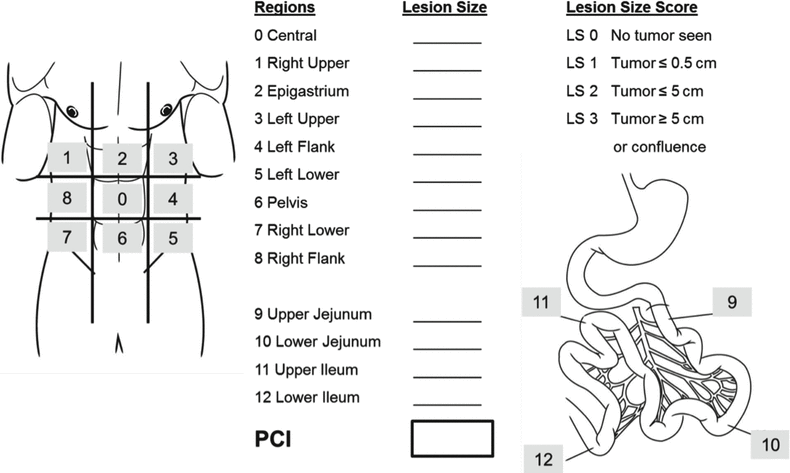
Peritoneal Cancer Index (PCI)
Key Concept: A key quantitative prognostic index is the peritoneal cancer index (PCI). Cytoreductive surgery should not be undertaken with curative in patients with PCI ≥20, as the results of CRS + HIPEC are not different than systemic therapy alone.
Presurgical extent of disease evaluation should provide reliable information about the tumor location, the extent of peritoneal tumor burden, and distribution and extent of the disease so that diligent patient selection can be carried out [3]. Studies on the preoperative clinical staging of PC have shown that the reliability of computed tomography (CT) for predicting the stage of the disease is somewhat limited [36]. As reviewed earlier [3], various scoring systems are currently in use for the assessment of peritoneal surface disease burden:
The PCI [37] is well established, currently in use at the major PSM centers worldwide (Fig. 5.3), and was confirmed as the preferred scoring system by a consensus conference held in Milan in 2006 [40]. Sugarbaker divides the abdominal compartment into nine regions (Regions 0 through 9), and the small bowel separately into four regions (Regions 10 through 13). After exploring the abdomen, all single regions are assigned a score corresponding to the greatest possible extent of tumor involvement by size of the largest peritoneal surface lesion within that region (lesion size from 0, no tumor seen, to 3, tumor >5 cm). Out of this, the maximum possible number of points in the PCI is thus 39, and the lowest is 0. Sugarbaker’s analyses revealed that patients with a PCI score of less than 20 have a reasonable likelihood of complete cytoreduction, thereby prognosis (in comparison with the previous approach of palliative chemotherapy alone and/or best supportive care) that may be favorably impacted by CRS + HIPEC. A challenge for the future will be reliable pre-therapeutic (before surgical exploration) prediction of tumor involvement of the small bowel and/or mesentery, as this represents one of the major limiting factors for the ability to achieve complete cytoreduction with CRS. The Society of Surgical Oncology has published surgical selection criteria for patients with PSM in 2006 [40].
Residual Tumor Classification (Completeness of Cytoreduction, CC Score)
Key Concept: Another key prognostic indicator is completeness of cytoreduction (CC) score. The goal is to attain complete removal of all grossly evident disease (CC0) or to leave behind only a few minute deposits of peritoneal surface tumor that can be treated effectively with HIPEC (CC1). Therefore, complete CRS implies both CC0 and CC1. The only way in which the patient can achieve long–term benefit is through having complete cytoreduction (CC0/1).
The major basis for prognosis in surgical oncology is completeness of resection, obtaining complete clearance of grossly apparent disease. This is usually determined by the R-classification (residual tumor classification). CRS is also based on the target criteria used in surgical oncology—achieving complete macroscopic and microscopic freedom from tumor (R0 resection). It is difficult to communicate in terms of R0 resection after multivisceral resection in the context of CRS. Therefore, the classification of “completeness of cytoreduction,” so-called CC classification [41], was developed and also affirmed at the 2006 consensus conference in Milan (Table 5.2) [40]. In patients with mucinous pseudomyxoma peritonei who undergo CRS + HIPEC, the R0 resection referred to elsewhere in the gastrointestinal tract is equivalent to CC 0 (no residual tumor) and CC1 status (<0.25 cm residual tumor tissue),whereas in invasive gastrointestinal tumors such as CRC and/or gastric carcinomas, R0 resection is only equivalent to CC 0 status. Completeness of resection is of paramount importance for patients with PC, and it has been clearly shown that patients with CC 0/CC 1 resections have a significantly improved survival period than those who do not [41–44]. In fact, there is no indication for CRS/HIPEC treatment in the setting of incomplete cytoreduction (CC2/3). Therefore, the CC classification is important not only in patient selection for CRS (only those in whom CC 0/1 status can be achieved should undergo attempted CRS) but also in estimating oncological outcome of CRS for a given CC score, which has been shown to be of significant prognostic value, serving as a surrogate marker for disease-free and overall survival after CRS for patients with PSM due to CRC [45, 46].
Table 5.2
Completeness of cytoreduction (CC) score
CC 0 | No residual tumor (= R0 resection) (en bloc resection) |
CC 1 | <0.25 cm residual tumor tissue (complete cytoreduction) |
CC 2 | 0.25–2.5 cm residual tumor tissue (incomplete cytoreduction with moderate residual tumor proportion) |
CC 3 | >2.5 cm residual tumor tissue (incomplete cytoreduction with high residual tumor proportion) |
HIPEC: Technique, Rationale, and Drugs
Key Concept: The strategic rationale for HIPEC includes increased chemotherapeutic agent concentration/dose at the intended site of action, increased cytotoxic effect of the administered intraperitoneal agent, reduced systemic absorption and toxicity of the chemotherapeutic, homogeneous distribution of intraperitoneal chemotherapy, and direct antitumor effect of hyperthermia.
Hyperthermic intraperitoneal chemotherapy (HIPEC) can be carried out as an open (“coliseum”) or closed procedure [3]. The coliseum technique allows manual distribution of the perfusate during HIPEC that is extremely important for certain anatomical regions. The principle is that the abdomen is initially filled with a carrier solution (dialysis or Ringer’s solution). The carrier solution is then passed through the HIPEC machine to heat it. Once a steady-state temperature of minimum of 42 °C has been reached (optimally a mean temperature of 43–44 °C), the chemotherapeutic agent is added and HIPEC starts. The intra-abdominal temperature is measured every minute, and patient-specific temperatures (bladder, head, esophageal, and/or rectal temperature probe temperature assessed by the anesthetist) are also closely monitored and recorded. After 30–90 min of HIPEC, the carrier solution is drained along with the chemotherapeutic agent, and the abdomen is lavaged with approximately 8–10 L of Ringer’s solution. Both the perfusate and lavage solutions must be disposed of as potentially hazardous waste material. Locoregional (intraperitoneal) administration of chemotherapy increases the local concentration of the chemotherapeutic agent at the site of action, the peritoneal surface. This reduces the systemic toxicity of the treatment, but at the expense of potentially increased postoperative morbidity related to the surgical procedure [47]. Some institutions create the anastomosis before and some after the administration of HIPEC. One animal study showed that anastomotic insufficiency is more likely to occur when systemic 5-fluorouracil (5-FU) treatment is carried out around the time of HIPEC than when locoregional chemotherapy is used alone [48]. Another study in a rat model showed that HIPEC consistently resulted in delayed healing of colonic anastomosis [49], raising the question whether technical modifications (e.g., proximal diversion) are indicated in the setting of HIPEC.
In HIPEC, the carrier solution (dialysis or Ringer’s solution) is initially heated to a temperature of 43 °C, with instillation of the chemotherapeutic agent only being carried out afterwards. The chemotherapeutic agent is circulated in the peritoneal cavity administered for 30–90 min, depending on the preference of the peritoneal carcinomatosis center concerned and the agent being utilized. When HIPEC has been completed at a mean temperature of 43–44 °C, the abdomen may be lavaged. Postoperatively, the patient is monitored in an intensive care unit. It is important to note that cisplatin-containing substances in particular can also have direct cardiotoxic effects. As a result of the large wound surface, it is possible for cis-diaminedichloroplatinum (CDDP) to be washed into the bloodstream, leading to cardiotoxicity, for which care in a monitored setting following operation is imperative.
At present, the agents used in HIPEC are mainly mitomycin C, cisplatin (CDDP), oxaliplatin, and doxorubicin. Intraperitoneal administration of chemotherapeutic agent achieves high response rates in patients with peritoneal carcinomatosis, as the peritoneum–plasma barrier makes it possible to administer high doses of the drug [50]. On the basis of analyses conducted during peritoneal dialysis, Dedrick et al. showed in 1978 that the peritoneal permeability of hydrophilic cancer drugs is lower than the known plasma clearance of the same agents [32]. The chemotherapeutic drugs mitomycin C, cisplatin, and/or oxaliplatin are the agents of choice for HIPEC. These drugs have a relatively high molecular weight (mitomycin C, 334 Da; cisplatin, 300 Da; oxaliplatin, 397 Da). Due to reduced permeability into the plasma through the peritoneal barrier, they consequently have lower systemic concentrations and thus lower associated toxicity [51, 52]. The challenge when interpreting the international literature is that there are also centers in which systemic chemotherapy is administered simultaneously with heated agents delivered into the peritoneal cavity [3]. Another important variable aside from the type (open versus closed technique) and duration (30, 60, 90 min) of HIPEC is the temperature at which the chemotherapy is delivered into the peritoneal cavity (generally >41.5 °C). Hyperthermia above 41 °C alone produces a direct antitumor effect. However, tumor cells react through upregulation of heat shock proteins, which may be able to produce some thermal tolerance [53]. This cytotoxic effect has been demonstrated only for drugs containing platinum [54] and for mitomycin C [55]. It is also important to recognize that hyperthermia itself has deeper tissue effects [56]. The rationale for hyperthermic delivery of intraperitoneal chemotherapy immediately after CRS is summarized in Table 5.3 [3]. Deeper tissue effects of HIPEC are discussed in the following section.
Table 5.3
Rationale for hyperthermic delivery of intraperitoneal chemotherapy immediately after colorectal surgery
Increased penetration of the chemotherapeutic agent into tissue |
Increased cytotoxic effect |
Cytotoxic effect of hyperthermia itself |
Reduced systemic toxicity of administered agent at higher concentrations |
Direct treatment of free intraperitoneal tumor cells |
Multimodal Therapy in Peritoneal Carcinomatosis
Key Concept: Multimodality therapy consisting of cytoreductive surgery + HIPEC in patients with CRC peritoneal carcinomatosis is superior over systemic therapy alone.
Published randomized phase III trials in PSM and CRC had been recently reviewed in detail [4]. In this and another recent review addressing the application of the second look operation [20], it was emphasized that systemic multidrug chemotherapy alone has not altered significantly the natural history and/or prognosis of patients with PSM and CRC. First-line 5-fluorouracil-based regimens (5-FU/leucovorin (LV) including oxaliplatin (FOLFOX) and irinotecan (IFL, FOLFIRI) with or without targeted monoclnal antibody therapy using bevacizumab (IFL/bevacizumab) or cetuximab (Erbitux) have increased response rates to a range of 25–55 % and median overall survival rates from 12 to 24 months compared to the benchmark regimen applied as the standard of practice over the past 40 years (5-FU or 5-FU/LV) [57–65]. A retrospective pooled analysis of over 2,000 study subjects enrolled in the North Central Cancer Treatment Group (NCCTG) Phase III Trials N9741 and N9841 demonstrated a median survival of 12.7 months in patients with peritoneal spread of CRC [66]. Treatment-adjusted analysis showed that patients with PSM and CRC have worse survival compared to patients with advanced CRC and distant metastases without PSM (p = 0.0006). Oncological outcome in patients with PSM of CRC origin treated by second line 5-FU + leucovorin + oxaliplatin (FOLFOX) was not significantly improved. Progression-free survival was ~6 months. This is in contradistinction to reported median survival rates between 19 and 63 months in experienced centers using CRS + HIPEC to treat limited PSM of CRC origin (that can be completely resected), underscoring the advantage of this multimodality therapeutic approach [19, 67–69]. Although FOLFOX was found to be superior to irinotecan + 5-FU/leucovorin (IFL) and irinotecan + oxaliplatin (IROX) as first-line therapy in the pooled analysis of the NCCTG trials by Franko et al., no survival benefit was apparent with second line use [66]. Systemic multidrug chemotherapy has not altered the natural history of peritoneal carcinomatosis as patients suffer disease progression and functional deterioration due to visceral obstruction, malignant ascites, and cancer cachexia over a limited median survival [4].
The multimodality therapy approach, using systemic chemotherapy plus aggressive CRS and HIPEC, has shown clearly promising results. The randomized controlled trial (RCT) of Verwaal et al. demonstrated a statistically significant survival advantage for this therapeutic approach [67, 68]. This was an RCT comparing CRS + HIPEC versus 5-FU-based systemic chemotherapy, which demonstrated a significant OS benefit with median survival of 22 months versus 12 months and 2-year survival of 44 % versus 22 %, respectively [67, 68]. The study also determined that ~5 patients must undergo CRS + HIPEC for one patient to experience survival advantage at 3 years.
Other studies have shown that patients with PC from CRC treated with chemotherapy alone have a median survival of 5–19 months, whereas those treated with CRS + HIPEC for early PC from CRC have reported median survival in the range of 48–63 months and 5-year survival of ~50 % following complete cytoreduction and HIPEC [4]. This data represents significant progress over the past 20 years for what was once thought to be a preterminal condition for which only palliative intervention was previously considered. It is also important to recognize what data is needed in order to further advance and optimize this multimodality treatment approach for PC of CRC origin. This is summarized in Table 5.4. One particular interesting consideration is that of neoadjuvant systemic therapy. Response to neoadjuvant therapy can provide important insights into the biology of disease, tumor response to treatment, and surgical decision making in terms of likelihood of achieving complete cytoreduction. Future clinical trials are likely to address this important unanswered question pertaining to the role of neoadjuvant therapy as part of multimodality treatment in PC from CRC [70].
Table 5.4
Unanswered questions in the multimodal treatment approach for peritoneal surface malignancy (PSM)
Chemotherapy preoperatively (neoadjuvant setting) followed by CRS + HIPEC versus |
CRS + HIPEC alone versus |
CRS + HIPEC + intraoperative systemic chemotherapy versus |
CRS + HIPEC followed by postoperative chemotherapy (adjuvant setting) versus |
Taking all 4 aspects into account: neoadjuvant + CRS + HIPEC + intraoperative chemotherapy + plus adjuvant chemotherapy |
Patient selection is critical in terms of maximizing oncological benefit of multimodality treatment, with the critical determinant being likelihood of achieving complete cytoreduction (CC 0/1). Clinical decision support systems (CDSS) based on specific clinical, pathological, biomarker, and patient data will ultimately facilitate risk stratification, further enable patient selection for CRS + HIPEC, optimize selection of high-risk patients for PC to undergo second look laparotomy, and individualize multimodality therapy in patients with PSM in CRC [71]. One major problem in patients with PSM of CRC origin is that approximately 50 % will have recurrence of disease after treatment [72, 73], which serves as the fundamental basis for performing a second look operation.
Second Look Concept
Key Concept: Second look laparotomy: …a new plan for early intervention in patients with high risk for local–regional recurrence after primary colon cancer surgery…The high incidence of prolonged survival in this group of patients with early definitive intervention supports the concept of maximal benefit in patients with minimal disease.
– Paul A. Sugarbaker
We have recently reviewed this in detail and will summarize the key points here [20]. Completeness of cytoreduction (CC0/1) and limited peritoneal surface disease (PCI <20) are associated with improved survival following CRS/HIPEC. Importantly, not only is survival improved after CRS + HIPEC for limited PC but also operative morbidity and mortality is significantly reduced because surgery is less extensive. Early peritoneal carcinomatosis is undetectable by conventional imaging or through the use of biomarkers; therein lays the challenge. Second look laparotomy followed by CRS + HIPEC data could only be generated thus far because some groups have performed the so-called second look laparotomy to identify patients that could potentially benefit from second CRS + HIPEC at a time when none of the patients had clinical or radiographic evidence of recurrent PSM [74–76]. The rationale for performing second look laparotomy (generally not laparoscopy, as this modality cannot expose all relevant planes of dissection to ascertain presence of and magnitude of PC) is to identify PSM of CRC origin early in the natural history of the disease in patients at high risk of having disease recurrence. The goal is to identify at-risk patients when tumor volume is below an important clinically detectable threshold, recognizing that completeness of cytoreduction is more readily attained when peritoneal surface disease is of limited extent (PCI <20), where the oncological impact of CRS + HIPEC conducted with curative intent is greatest [37]. As pointed out before [20], the concept of second look operation in cancer is over 60 years old, was probably established in 1948, and first described by Wangensteen in 1949 [5, 77, 78]. Different groups studied the “second look approach” in different tumor types for various indications: cancer staging, palliative treatment in cancer recurrence, and other non-cancer-related diseases, such as mesenteric artery occlusion and in postoperative complication algorithms [5, 27, 72–105]. Esquivel and Sugarbaker investigated a large number of patients with PSM of appendiceal origin during a 12-year period [5, 74]. Out of 321 patients, 98 patients (31 %) underwent second look procedure followed by CRS + HIPEC. The overall 5-year survival rate in these 98 of 321 patients was 74 % compared to 68 % in the remaining 223 of 321 patients. These data clearly show that there is a subpopulation of patients that may benefit from follow-up second look laparotomy and CRS + HIPEC. On the other hand, symptomatic patients, who present with bowel obstruction as a symptom or have a large amount of tumor (PCI >20), have significantly worse survival; hence, patients with a high amount of tumor load have questionable benefit from either second look laparotomy or CRS + HIPEC. In fact, there is no overall survival benefit when CRS is undertaken for patients with PCI exceeding 20 [37]. Maggiori et al. investigated 41 patients with PSM of CRC origin who underwent second look operation and who had no clinical or radiomorphological sign of recurrence at the time of second look. Over half of the patients (23/41, 56 %) underwent subsequent CRS + HIPEC [76]. The reported 5-year overall survival rate was 90 % and 5-year disease-free survival, 44 %. An important finding in this study was that early peritoneal surface recurrence of CRC could be identified absent clinical or radiomorphological signs of disease at a time in its natural history when the oncological benefit of CRS + HIPEC could be maximized. Importantly at-risk asymptomatic patients can be diagnosed with PSM over 50 % of the time. Sugarbaker focused on clinical parameters to identify these at-risk patient in an effort to improve selection and provide clinical decision support to the surgical oncology community; he published suggestions for guidelines for second look operation [72, 73] The major aim of second look operation is to achieve complete tumor resection (R0 resection, CC 0/1 resection). The limited extent of PC that may be identified during the second look in asymptomatic patients lends itself to completeness of tumor resection, estimation of prognosis, and positively impacting patient outcomes through multimodality therapy, CRS + HIPEC. Therefore, patients with limited local–regional recurrence may have more benefit compared to possibly symptomatic patients with a high tumor burden, PCI. Recently, a group of experts in PSM suggested decision support algorithms for patients presenting for the first time with CRC and for those with recurrent CRC or already scheduled for programmed second look operation, which are discussed in the following section. Patients considered at risk for peritoneal carcinomatosis that may benefit from second look laparotomy include patients with perforated primary tumors (iatrogenic or spontaneous), completely resected synchronous limited PC at initial operation, synchronous ovarian metastases, and possibly T4 lesions that required adjacent organ resection and emergency presentation for obstructing/bleeding lesions that underwent surgery.
Decision Making/Preoperative Work-up
Indications and Interdisciplinary Tumor Board
Key Concept: An important element in patient selection for CRS + HIPEC is careful evaluation of the diagnosis and stage of disease as well as resectability of the peritoneal surface malignancy and operability of the patient; the findings of diagnostic testing must be reviewed by an interdisciplinary tumor board in order to arrive at an individualized plan of care.
A patient-centered, integrated, comprehensive, and evidence-based team approach is a “must” in individual cancer therapy. This individualized care approach to patients afflicted by cancer demands that each patient is carefully evaluated, and the findings of diagnostic testing reviewed collectively by a team that in the venue of an interdisciplinary tumor board arrive at an individualized plan of care. All prior patient reports of any treatment intervention, histopathological review, laboratory parameter dynamics during multimodal treatment, and radiomorphological imaging are mandatory elements requiring review by the team prior to treatment recommendations, which must take into account available best level evidence. In addition to such team members as surgical, medical, and radiation oncologists, radiologists, geneticists, pathologists, psychologists, rehabilitation specialists, nurses, as well as students should be involved as part of the interdisciplinary tumor board. An example of an interdisciplinary tumor board structure is shown in Fig. 5.4. Interactions with external stakeholders in academia, administration, and government are shown.
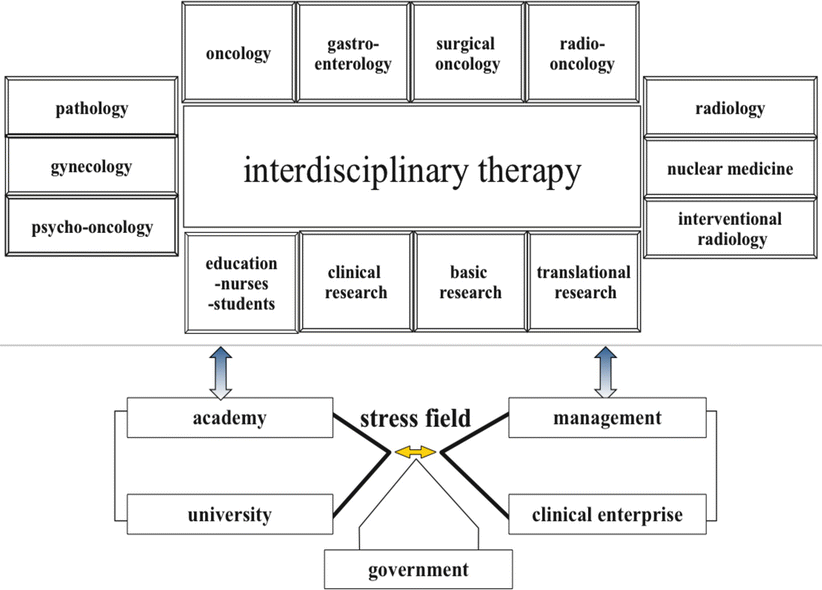

Fig. 5.4
Interdisciplinary tumor board including interactions with academia, administration, and government
The indications for CRS + HIPEC in patients with PSM have been reviewed by several authors [27, 96] and are shown in Table 5.5 [96]. Factors to consider in patient selection for CRS + HIPEC include disease-free interval; extra-abdominal metastases; extent of liver metastases; histology of the primary tumor; local–regional tumor burden (PCI); expected completeness of cytoreduction (CC0/1); patient age, comorbidity, and performance status; carcinomatosis-related complications (SBO, ascites); and prior systemic therapy (toxicity, resistance). A recent expert review of CRS + HIPEC for CRC [20] suggested two clinical decision support algorithms for patients presenting with a diagnosis of CRC (Fig. 5.5) and those who present with CRC recurrence or are already planned for programmed second look laparotomy (Fig. 5.6).
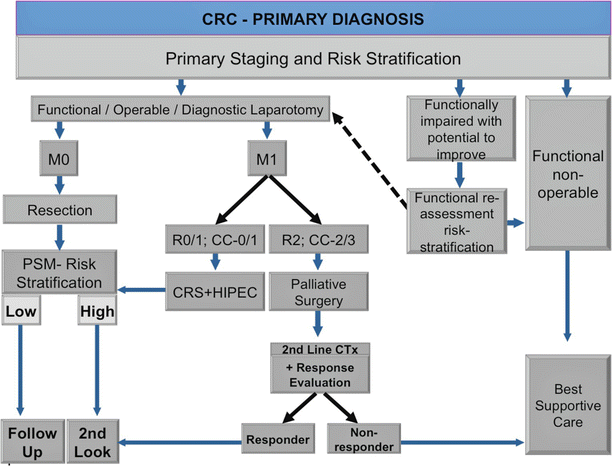
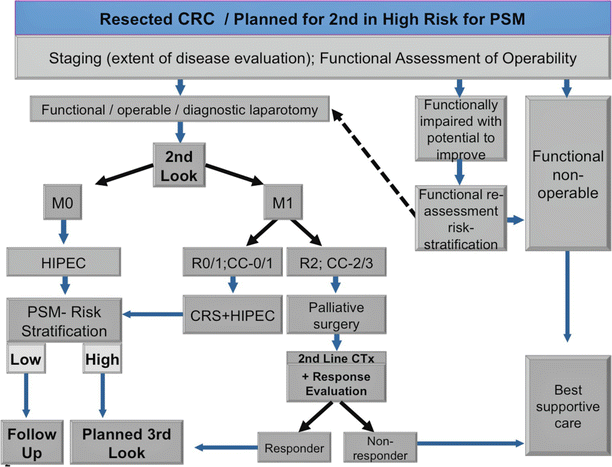
Table 5.5
Indications for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC)
PSM | Indications |
|---|---|
Primary peritoneal neoplasms | Diffuse malignant peritoneal mesothelioma (epitheloid type) |
Well-differentiated peritoneal mesothelioma | |
Multicystic peritoneal mesothelioma | |
Papillary serous primary peritoneal mesothelioma | |
Primary peritoneal adenocarcinoma | |
Secondary peritoneal neoplasms | Gastrointestinal carcinoma (appendix carcinoma, CRC, small bowel carcinoma, gastric carcinoma, pancreas carcinoma) |
Gynecological/urogenital tumors (e.g., epithelial ovarian cancer) | |
Other rare primary tumors with potential peritoneal metastasis (e.g., malignant melanoma, breast cancer, cervix carcinoma, bladder carcinoma) |

Fig. 5.5
Algorithm for patients with primary CRC at time of primary diagnosis including PSM risk stratification (Modified from: Brücher et al. [20])

Fig. 5.6
Algorithm for patients with CRC, who had been scheduled for second look operation and/or who present with recurrence (Modified from Brücher et al. [20])
Contraindications
Key Concepts: Contraindications to CRS + HIPEC include but are not limited to patient with inability to tolerate the operation (poor performance status), PCI >19, prohibitive medical comorbidities, extra–abdominal metastases, massive retroperitoneal tumor involvement and/or root of mesentery invasion, extensive small bowel disease, >3 liver metastases, and aggressive biology (high grade, signet ring cell).
These can be divided into absolute and relative contraindications [3] (Table 5.6). CRS + HIPEC can only provide survival benefit in patients having good performance status, limited peritoneal surface disease, and those in whom complete cytoreduction is highly likely. Thus, cytoreductive surgery and HIPEC should not be pursued in patients with poor performance status (Karnofsky <70), weight loss ≥10 %, unremitting pain; carcinomatosis-related morbidity (ascites, SBO involving >1 SB segment); prohibitive medical comorbidities (cardiac, pulmonary, renal, hepatic, florid infection); extra-abdominal metastases; massive retroperitoneal involvement or root of mesentery invasion by tumor; extensive small bowel disease (high risk of short-bowel syndrome if resected); unresectable peritoneal disease (PCI ≥20); or aggressive biology (high-grade, signet ring). It is important to note that liver (≤3) metastases and peritoneal disease progression while on chemotherapy are not contraindications for CRS + HIPEC so long as complete cytoreduction can be achieved. CRS is contraindicated in patients with PCI >19, as median survival is no different after CRS/HIPEC than that obtained with systemic therapy alone (~18 months). These decisions in selecting patients for CRS + HIPEC with curative intent are best made in centers of excellence with multidisciplinary teams devoted to the care of patients with PSM.
Table 5.6
Absolute and relative contraindications to cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC)
Absolute contraindications |
Massive involvement of the retroperitoneum |
Invasion of the mesenteric pedicle |
Massive small-bowel involvement (that would result in a short bowel after radical resection) |
Unresectable intra-abdominal and/or extra-abdominal metastases |
Incurable second malignancy |
Karnofsky index <70 |
Relative contraindications |
High body mass index |
Cardiac contraindication |
Hepatic contraindication |
Renal contraindication |
Florid infection |
Acute ileus |
Quantitative Prognostic Factors (QPIs)
Key Concept: Cross–sectional and functional imaging with CT and CT/positron emission tomography [PET]) is the first-choice diagnostic test in the work–up of peritoneal carcinomatosis; however, these modalities often underestimate preoperative PCI necessitating laparoscopic or open laparotomy staging of extent of disease in order to determine likelihood of CC0/1.
Clinically very important are quantitative prognostic indicators (QPIs) [3], although the quality of the evidence supporting their use in clinical practice varies from one tumor entity to another and high-level published evidence is sometimes lacking. No data are available on tumor markers as qualitative prognostic markers in PSM. With regard to histopathology, the only available data show that patients with poorer differentiation (high-grade, signet ring cell) have worse prognosis than those with well/moderately differentiated cancers. The value of preoperative cross-sectional imaging (CT, MRI) appears to be limited to patients with mucinous PSM. Our own research on the use of preoperative 18F-fluorodeoxyglucose-positron emission tomography and computed tomography (FDG-PET/CT) scanning in comparison with the intraoperative PCI score shows that it has prognostic value [36]. The Sugarbaker PCI score (P < 0.0001) and CC score (P < 0.001) are both clinically relevant prognostic factors in PSM of CRC origin [41].
Ethical Considerations
Key Concept: We must do our best to inform our patients and to enhance their comprehension about their disease and prognosis; most importantly to communicate to them our best estimate of likelihood of cure of their disease.
Independent of the underlying cancer leading to PSM, our society has a kind of Zeitgeist: that peritoneal carcinomatosis means “death soon.” This follows decades of therapeutic nihilism for this stage of cancer. Treatment of patients who suffer from peritoneal carcinomatosis is a burden for both patient and provider, for it is a formidable problem and the treatment is extensive in nature and burdensome itself. This was, is, and always will be a situation that tests our forbearance, our resolve, and at times our faith, as we are often confronted at times with malignancy and intervene at the crossroads of potentially curative and palliative treatment in the face of incompletely defined tumor biology. Combating PSM means being aware about areas of potential ethical conflict: informed consent, treatment refusal, treatment waiver, decision-making ability, capacity to consent, truth at the bedside, truth in the OR, the ICU, confidentiality, research on patients, termination of life-sustaining measures, preserving hope while communicating the actual implications of clinical findings, among others. Dealing with the diagnosis of PSM means to be aware that we must often confront life-limiting challenges. The philosopher Epikur (341–270 ante Christi) stated “Ars moriendi ars vivendi” meaning the art of dying is the art of living. This refers to the process of how to die well and can lead one to conclude that terms such as palliative care, supportive care, or terminal care are second rate and inconsistent with that ethos. Ethics has as one of its main tenets that humans have the freedom to decide. It has been shown that patients with advanced malignancy are willing to accept high-risk interventions and toxic treatments for a slight (even 1 %) chance of cancer cure; at the same time, most patients would not accept such therapy without cure, even if it may significantly increase anticipated survival [97]. A recent study of patients participating in the Cancer Care Outcomes Research and Surveillance (CanCORS) study found that over 80 % of those with CRC did not report understanding that chemotherapy was unlikely to cure their cancer. The authors concluded that “many patients receiving chemotherapy for incurable cancers may not understand that chemotherapy is unlikely to be curative, which could compromise their ability to make informed treatment decisions that are consonant with their preferences” [97]. It is our ethical obligation as human beings and physicians to do our best to inform our patients and to enhance their comprehension about their disease, even if the patient’s satisfaction with the health-care provider and or system is negatively impacted.
Intraoperative Work-up
Cytoreductive Surgery: Logistics, Strategy, and Technique
Key Concepts: High–voltage electrosurgery is utilized for cytoreduction of peritoneal surface malignancy, thereby generating a significant amount of smoke during the procedure which necessitates the use of proper operating room ventilation and a smoke evacuator system used continuously over the surgical field. Heated intraperitoneal chemotherapy is safe for the surgical team and operating room personnel as chemotherapy exposure is negligible, particularly with adherence to universal precautions, and environmental/individual protective measures.
Cytoreductive surgery is a major operation including multiple visceral resections and stripping of peritoneal surfaces. Complex surgical maneuvers such as liver mobilization or full exploration of the omental bursa including the upper recess (the area between the right crura of the diaphragm, liver, and vena cava) and the foramen of Winslow are mandatory to establish CC-0/1 [98]. Therefore, even in the face of limited peritoneal surface disease, cytoreduction is considered a complex abdominal operation and requires a dedicated team and adherence to a comprehensive, standardized preoperative preparation protocol. The HIPEC procedure puts the operating room (OR) and intensive care unit (ICU) personnel within unfamiliar territory at outside their proverbial “comfort zone.” Even in high-volume cancer centers, handling and delivering cytotoxic agents is not a routine in most ORs. Therefore, careful planning and detailed preparation, transport, administration, disposal, and safety protocols should be followed in order to avoid errors risking the patient or OR staff.
Preoperative planning is conducted in two levels. The first level is oncological and the second level is technical.
Oncological Planning
Oncological planning was outlined before (“Indications”) and includes:
(a)
Indication for surgery (disease type, disease status, PCI)
(b)
Lack of contraindications (extraperitoneal disease, PCI >20, >3 liver metastases, poor performance status)
(c)
Surgical history (prior surgical procedures for PSM or resection of primary tumor)
(d)
Oncological history (date of diagnosis, age at diagnosis, stage at primary diagnosis, prior treatments delivered, and response evaluation)
In most centers this is done in a tumor board setting and discussed by a multidisciplinary team. In patients that are found to be eligible for CRS + HIPEC, the HIPEC protocol is decided upon and the patient is then scheduled for surgery.
Technical Planning
This is done by a dedicated team including surgical oncologist, anesthesiologist, ICU specialist, medical oncology, OR nurse, nutrition nurse, stoma nurse, pharmacy, and perfusionist.
The procedure is planned according to the following parameters:
Surgical Planning
Type of Disease
Diseases such as disseminated peritoneal adenomucinosis (DPAM) or benign cystic mesothelioma tend to adhere to organs and not to penetrate into the tissue; therefore, they require less visceral resections and result in less surgical trauma and consequent operative morbidity. Other diseases such as serous papillary adenocarcinoma of the ovary or adenocarcinoma of the colon are more likely to penetrate into organs and tissues and as a result require more visceral resections, and the extent of surgical trauma and attendant morbidity are higher [99].
Extent and Location of Disease
The complexity of the procedure, its success, and the rate of postoperative complications are highly correlated with extent of disease as measured by PCI [100]. Volume of disease and location of disease require careful consideration for detailed surgical and anesthetic planning as they may impact postoperative course and recovery. For example, large volume of disease located between the right lobe of the liver and right diaphragm requires liver mobilization and retraction that may result in periods of low blood pressure as a result of vena caval compression. Full stripping of the diaphragm requires the insertion of a chest drain in order to avoid postoperative pleural effusions. Another example is tumor in the abdominal wall. Disease recurrence in surgical scars is common in patients with PSM [101]. When abdominal wall tumor masses exist, careful surgical planning of abdominal wall resection and reconstruction is required.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree