Capsule endoscope
Manufacturer
Dimensions
Field of view
Image capture
Battery life
Data transmission
PillCam SB
Given Imaging, Yoqneam, Israel
11 × 26 mm
156°
2-6 fps
12 h
Radiofrequency
Endocapsule MAJ-1469
Olympus Medical Systems Corporation, Tokyo, Japan
11 × 26 mm
145°
2 fps
8 h
Radiofrequency
OMOM
Jinshan Science and Technology Co., Chongqing, China
13 × 27.9 mm
140°
2 fps
8 h
Radiofrequency
MiroCam
IntroMedic Co., Ltd., Seoul, Korea
10.8 × 24.5 mm
170°
3 fps
12 h
Electrical field propagation
CapsoCam SV1
Capsovision, Saratoga, USA
11 × 31 mm
360°
20 fps × 2 h then 12 fps
15 h
Capsule retrieved then USB download
PillCam Patency Capsule
Given Imaging, Yoqneam, Israel
11 × 26 mm
n/a
n/a
n/a
n/a
PillCam Colon
Given Imaging, Yoqneam, Israel
11 × 32 mm
172°
6 fpm in stomach
4–35 fps in SB
10 h
n/a
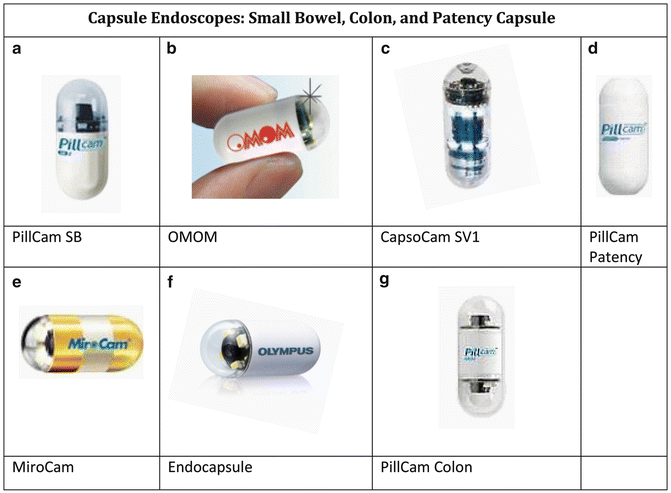
Fig. 7.1
Images of capsule endoscopes: small bowel, colon, and patency capsule
Preparation
Adequate inspection of the small bowel mucosa depends on a quality bowel preparation, as the capsule does not have the ability to clear debris from the lumen. Currently, there is no standard bowel preparation, nor is there an established scoring system to compare the quality of the small bowel preparation. A combination of a clear liquid diet and fasting, the use of osmotic laxatives and medications to stimulate peristalsis, have all been used to prepare the small bowel mucosa for CE. One meta-analysis of eight studies demonstrated superior mucosal visualization when the bowel was prepared with sodium phosphate (Na-P), polyethylene glycol (PEG) or erythromycin prior to the capsule compared with clear liquid diet alone [9]. A second meta-analysis of 12 studies demonstrated improved diagnostic yield of CE when patients were treated with an osmotic laxative prior to CE compared with those patients who received a clear liquid diet alone (odd ratio [OR] 1.81; 95 % confidence interval [CI], 1.25–2.63; p = 0.002) [10]. A third meta-analysis of eight randomized controlled trials confirmed an improved diagnostic yield for CE when PEG-based bowel preparations were used prior to capsule deployment (OR 3.11; 95 % CI = 1.96–4.94; p <0.0001)[11]. Interestingly, sub-group analysis did not demonstrate a benefit of Na-P preparations compared to fasting alone (OR 1.32; 95 % CI = 0.59–2.96; p < 0.0001). The volume of the bowel preparation required has also been evaluated. A prospective randomized study compared 2 and 4 l of PEG solution in 201 patients undergoing CE for gastrointestinal bleeding, abdominal pain or suspected CD. The 2-l preparation demonstrated equal efficacy to the 4-l preparation in terms of mucosal visualization, capsule completion rate and identification of small bowel pathology, suggesting the 2-l preparation was adequate for CE. [12] There is no standard method to report the quality of the small bowel preparation in capsule endoscopy. A score has been proposed using the proportion of mucosa visualized on images, and a quantification of the degree of obscuration of the mucosa by bubbles, debris or bile. [13] The inter-observer agreement of this method was excellent in one study (k = 0.8), although the system has yet to be validated prospectively. To date, there is no consensus regarding the standard method to prepare the small bowel for CE, although expert consensus favors the use of some type of preparation.
CE in Suspected Crohn’s Disease
Crohn’s disease is a chronic inflammatory disorder that may affect any segment of the intestinal tract. Up to 90 % of patients will have involvement of the terminal ileum and colon at diagnosis or in follow-up, and ileocolonoscopy is adequate to make the diagnosis [14–16]. However, in a subset of patients, mural inflammation is confined to the proximal small intestine, out of the reach of the standard colonoscope. [4, 14, 17] To adequately evaluate for the presence of CD in this subgroup of patients, a more thorough assessment of the small bowel is required. Traditionally, small bowel follow-through (SBFT), occasionally augmented by push enteroscopy or ileoscopy has been utilized to evaluate for small intestinal disease. Unfortunately, SBFT has limited ability to detect mild mucosal inflammation found early in the course of CD, and in particular, for disease confined to the proximal small bowel [4, 18, 19]. Push enteroscopy and ileoscopy are limited in their scope, leaving the majority of the small bowel mucosa out of reach. This difficulty in evaluating the small bowel adequately likely explains in part the historical delay in the diagnosis of CD between 1 and 7 years from symptom onset [20, 21].
The advent of capsule technology has facilitated the evaluation of suspected small bowel CD, allowing for more thorough assessment of small bowel mucosa along the entirety of its length, including the segments previously inaccessible via push enteroscopy and ileoscopy (Fig. 7.2, Videos 7.1, 7.2, 7.3, and 7.4). The technology appears to have additional diagnostic yield of up to 70 % for CD isolated to the small bowel following a negative ileocolonoscopy [4, 18, 22]. In a study of 80 patients with suspected CD completing CE, SBFT and ileocolonoscopy, CE demonstrated superiority to SBFT in the detection of inflammatory lesions, with the combination of CE and ileocolonoscopy identifying 97 % of all inflammatory lesions, and SBFT and ileocolonoscopy detecting only 57 %. Of the patients diagnosed with CD, 55 % were diagnosed based on CE findings alone. [4] Ileocolonoscopy demonstrated similar diagnostic yield to CE for the identification of lesions in the terminal ileum and cecum. Whereas ileocolonoscopy detected most of the cecal inflammatory lesions, CE identified the majority of lesions confined to the terminal ileum, suggesting these two modalities are complementary in the evaluation of suspected CD, although the study recognized that ileocolonoscopy should be the first test in the evaluation of suspected CD (Fig. 7.3).
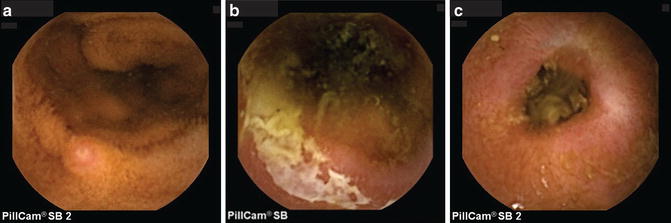
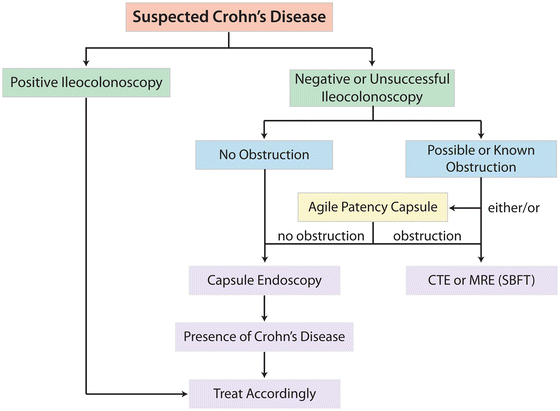

Fig. 7.2
Small bowel capsule endoscopy findings in inflammatory bowel disease. (a) aphthous ulcer; (b) ulcer with exudate; (c) small bowel stricture

Fig. 7.3
Capsule endoscopy in the evaluation of suspected Crohn’s disease. CTE computed tomography enterography, MRE magnetic resonance enterography, SBFT small bowel follow-through. Adapted from Mergerner K, Ponchon T, Gralnek I, Pennazio M, Gay G, Selby W, et al. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Endoscopy 2007; 39(10):895–909
Commensurate with the development of the video capsule endoscope has been the advancement in techniques of cross-sectional imaging, namely CT enterography (CTE) and MR enterography (MRE). Both modalities have demonstrated improved sensitivity for the detection of small bowel pathology, leading to multiple proposed algorithms in the evaluation of IBD. CTE and MRE have the advantage over CE of providing information regarding transmural inflammation, and the ability to detect the presence of extraluminal disease such as lymphadenopathy, abscesses or fistulae [17]. CE may be superior, however, in detecting superficial mucosal ulcerations found early in the course of CD. Another limitation to the use of CTE and MRE is the need for large volumes of oral contrast for adequate visualization of the small bowel, which may be difficult for patients with diminished oral intake or partial small bowel obstruction. In addition, small bowel wall thickening may be overestimated if the intestinal lumen is not adequately distended with oral contrast during the study. CTE is more widely available than MRE and is less expensive; however, CTE requires a considerable radiation exposure, which becomes a particular issue in younger patients who may require repeated examinations. MRE may have the advantage over CTE in the ability to differentiate in some instances between fixed fibrostenotic lesions and potentially reversible inflammatory strictures [23, 24]. The diagnostic yield of CE, CTE and MRE was compared in a meta-analysis of 12 trials involving 428 patients with suspected CD [22]. CE demonstrated incremental yield over that of SBFT (32 %, p < 0.001, 95 % CI 16–48 %), ileocolonoscopy (22 %, p = 0.009, 95 % CI 5–39 %) and CTE (47 %, 0 < 0.001, 95 % CI 31–63 %). In this study, CE did not demonstrate clear superiority to MRE, although the number of patients in this subgroup were small (n = 31). A subsequent study of 93 patients with suspected or newly diagnosed CD evaluated this issue further [25]. Patients underwent ileocolonoscopy, CTE or MRE, followed by CE if no evidence of stenosis was identified on the preceding endoscopic and radiographic studies. The sensitivity and specificity for the diagnosis of CD of the terminal ileum were 100 % and 91 % for CE, compared with 81 % and 86 % for MRE, and 76 % and 85 % for CTE. Of note, 25 % of patients were excluded from CE due to stricturing disease, suggesting that preceding small bowel radiography or use of a patency capsule in some instances may be warranted to reduce the risk of capsule retention.
One detractor from the yield of CE in IBD patients is the potential lack of specificity of some of the mucosal abnormalities identified, due to a lack of histology confirmation, and lack of diagnostic criteria to define CD by video capsule. Mucosal erosions and ulcerations may be associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and have been identified in asymptomatic individuals in the absence of IBD [26–28]. To improve the positive predictive value of CE findings in suspected CD, a thorough history including recent ingestion of NSAIDs should be taken. Other conditions associated with small bowel ulceration include celiac disease, infections, ischemia, autoimmune enteropathy, and lymphoproliferative disorders. Regarding diagnostic criteria to establish a diagnosis of CD on CE, Mow et al. proposed the criterion of three or more ulcerations, identified in the absence of NSAID use for CD diagnosis. Ulcers were further defined as “white lesions within a crater,” with surrounding erythema, to be distinguished from erosions that were “white lesions with surrounding erythema” in the absence of mucosal depression [29]. The development of a validated scoring system of mucosal changes to diagnose small bowel CD may improve the specificity of CE in future study.
Capsule Endoscopy Scoring Systems
Two endoscopic scoring systems describing the severity of CD have been validated in ileocolonoscopy but not in CE. These include the Crohn’s Disease Endoscopic Index of Severity (CDEIS) [30], which grades both ulcerations and stenosis, and the Simple Endoscopic Index of Severity (SES-CD)[31], which grades both ulcerations (depth and/or diameter) and luminal stenosis. For capsule endoscopy findings, there are two scoring systems available to describe the extent and severity of small bowel inflammation in CD. The Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI) developed by Gal et al. in 2008 [32] measures inflammation on a 6-point scale ranging from hyperemia to large ulceration, extent of disease on a 4-point scale whether focal or diffuse, and the presence of one or more strictures, as identified in either proximal or distal small bowel segments. The score ranges between 0 and 36, the latter representing the most severe of disease activity [32]. The CECDAI has exhibited an excellent inter-observer agreement amongst reviewers (k = 0.87)[32] and has been validated in a multicenter prospective study [33]. The Lewis Index, in contrast, quantifies villous edema, mucosal ulceration, and luminal stenosis [34]. A score of < 135 was designated as normal or clinically insignificant, whereas a score of >790 was deemed to represent moderate to severe mucosal inflammation. In a retrospective study of 56 patients undergoing CE for suspected CD, only 12.1 % of patients with a score of <135 were diagnosed with CD, compared with 82.6 % of those patients with a score of >135 [35]. Both scores can be used to measure the degree of mucosal inflammation identified on CE, and therefore be used to describe severity of disease at initial diagnosis as well as evidence of mucosal healing following treatment. Although these scoring systems were devised to standardize CE reporting, their accuracy in measuring mucosal inflammation is not clear. A study comparing levels of fecal calprotectin (FC), an accepted marker of mucosal inflammation, [36] did not demonstrate good correlation between the CECDAI and FC, and the Lewis score only correlated for those patients in whom FC was low, that is, a low FC predicted a negative CE study [36, 37]. Higher levels of FC did not correlate with higher scores of inflammation on CE [37, 38]. Theories regarding the discordant findings include a heterogeneous population of patients with only 12 patients ultimately diagnosed with CD, a significant delay between FC measurement and CE in some cases, and the fact that the scoring systems do include non-inflammatory findings such as luminal stenosis to characterize disease severity [38]. Another study comparing the CECDAI with levels of C reactive protein (CRP), another accepted serological marker of inflammation in IBD, demonstrated only fair correlation an r=0.58 is actually fairly good correlation (r = 0.58, p < 0.01). [39] At this time, it seems that the CECDAI and Lewis Scores may be tools with which to compare CE findings amongst patients and in single patients over time, although they may be a complement, rather than a replacement for other markers of inflammation. Also, it is important to note that these scores are not able to discriminate between etiologies of mucosal inflammation; i.e., differentiate CD from NSAID enteropathy.
Comparison of CE and Advanced Endoscopic Techniques
Endoscopic techniques to evaluate the small bowel have included push enteroscopy, ileoscopy, and, in more recent years, device-assisted enteroscopy. Push enteroscopy and ileoscopy are limited to the proximal jejunum and terminal ileum respectively,. The advent of device-assisted enteroscopy has provided the opportunity for complete endoscopy of the entire small bowel, although this modality requires particular expertise to perform, is time consuming, and often requires anesthesia assistance. In addition, usually two separate procedures are required, with antegrade and retrograde approaches to achieve complete endoscopic evaluation of the small bowel. CE, in contrast, is non-invasive, and offers a method to visualize the entire small bowel in one procedure. CE and double balloon enteroscopy (DBE) have comparable yields in diagnosing small bowel CD. One meta-analysis of 11 studies comparing CE and DBE in 375 patients with suspected small bowel disease measured a diagnostic yield of 57 % with CE and 60 % with DBE [40]. Detection of small bowel inflammation was also comparable, with diagnostic yield of 16 % with CE and 18 % with DBE. In cases of capsule retention, deep enteroscopy techniques may be utilized to retrieve the capsule. Deep enteroscopy also offers the opportunity for tissue diagnosis in patients with positive CE, or may be offered to those patients with suspected or documented small bowel strictures in whom CE is contraindicated. Of the two modalities, CE would be recommended as the initial test of choice in the evaluation of possible small bowel CD, as it is noninvasive and allows for complete endoscopy, followed by deep enteroscopy if needed to obtain tissue diagnosis, with antegrade or retrograde approach dictated by CE findings.
CE in Established CD
As an imaging modality with high sensitivity for small bowel inflammation, CE is complementary to standard ileocolonoscopy and upper endoscopy in the evaluation and management of patients with established CD, affecting medical and surgical decision-making. CE can assist in documenting the extent and severity of CD, particularly in patients with persistent or unexplained symptoms [29, 41]. In a prospective study of 28 patients with persistently symptomatic CD, CE identified active inflammation in 82 % of patients compared with only 49 % detected by ileocolonoscopy, demonstrating an incremental yield of 33 %[20]. In a second study of 108 patients with established CD who underwent CE and CTE, 56 % were noted to have jejunal ulcerations not identified on cross-sectional imaging. It is important to note that the presence of jejunal ulcerations in this group was the only risk factor to predict relapse during 6 months of follow-up, and the CE study led to a modification in treatment plan in 20 % of patients [42]. Dussault et al. [43] also described medical decision-making following CE in a prospective study of 71 CD patients. CE was associated with medication changes and/or surgical treatment in 54 % of patients in the 3 months following CE, lending support to the concept of CE as integral to the evaluation and continued management of patients with established CD.
In addition to describing the extent of disease, CE may be useful as well in assessing for mucosal healing once therapy has been initiated (Fig. 7.4) [6, 41, 44]. Legnani and Abreu [41] documented healing of small bowel mucosal ulcers following biologic therapy in a patient with established CD. In a prospective study of 40 patients presenting with a CD flare, Efthymiou et al. [45] demonstrated that CE performed before and after treatment was able to document a significant reduction in the number of large ulcers identified in the small bowel. Mucosal healing of large ulcers correlated with clinical improvement measures such as: the Crohn’s Disease Activity Index (CDAI), the Inflammatory Bowel Disease Questionnaire (IBDQ), and C reactive protein (CRP) values in this study.
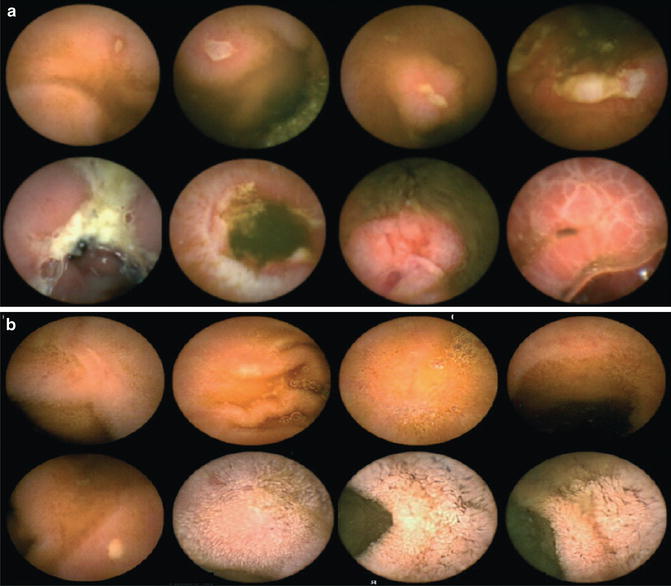

Fig. 7.4
(a) Capsule endoscopy images in inflammatory bowel disease. (b) Documentation of mucosal healing. Reprinted with permission from Calabrese C, Gionchetti P, Rizzello F, Liguori G, Gabusi V, Tambasco R, et al. Short-term treatment with infliximab in chronic refractory pouchitis and ileitis. Aliment Pharmacol Ther. 2008 May;27(9):759–64
Lastly, CE offers a non-invasive alternative, or a complement to ileocolonoscopy in the evaluation of postoperative recurrence of CD. Endoscopic recurrence of mucosal inflammation has been reported in the neoterminal ileum in 73–93 % of patients within 1 year of ileocolonic resection for CD [46]. Endoscopic recurrence often predicts symptomatic recurrence, and may impact future treatment plans. [17, 47, 48] Traditionally, postoperative evaluation of CD patients involved ileocolonoscopy alone. CE has demonstrated increased yield compared to ileocolonoscopy in identifying postoperative recurrence of CD in the neoterminal ileum following ileocolonic resection [47, 48]. CE may be particularly useful in cases where the surgical anastomosis is not readily accessible by endoscopy.
It is important to note that CE is considered complementary to CTE or MRE in CD, which can visualize transmural inflammation, and extraluminal disease, such as the presence of abscesses or fistulae. In addition, the potential for capsule retention must be considered in those patients in whom small bowel stenosis is identified on radiographic imaging. In cases where stricturing disease is suspected, the patency capsule should be considered prior to capsule endoscopy.
Utility of CE in Cases of IBD Unclassified
Population-based studies have demonstrated that in 4–10 % of adult patients diagnosed with colonic IBD, a distinction between UC and CD cannot be made following standard ileocolonoscopy, biopsy, and small bowel radiology. [5, 49, 50] Establishing the correct diagnosis has important implications for both medical and surgical treatment options as well as expected prognosis. The term “indeterminate colitis,” which was initially coined in 1978 to describe such patients in whom diagnosis remained unclear even following colectomy, has been replaced by the term “IBD unclassified (IBDU)” and refers to those patients in whom inflammation is confined to the colon but unclear as to phenotype following the standard evaluation described previously [5, 51]. By providing direct visualization of the entire small bowel, CE may play a role in further defining extent of mucosal involvement in IBDU, ruling out small bowel involvement in cases suggesting UC, or identifying small bowel ulcerations and consistent with underlying CD. [17] Several small studies have demonstrated CE as providing additional information to distinguish CD from UC [29, 52–54]. These studies demonstrated small bowel findings on CE, which changed the diagnosis in 29–40 % of patients [3]. It is important to note, however, that a negative CE does not preclude a future diagnosis of CD. In one study of 25 patients with IBDU and a negative CE, five patients were eventually diagnosed with CD in follow-up. [53]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








