Chapter 49 Cancer of the gallbladder
Overview
Gallbladder cancer is rare and traditionally has been considered an incurable disease with an extremely poor prognosis. The pessimism and nihilism that historically have been associated with gallbladder cancer stem from its commonly late presentation, often with disseminated disease, its overall dismal prognosis, and lack of effective therapy. The quote that sums up this sentiment best comes from Blalock, who stated in 1924, “In malignancy of the gallbladder, when a diagnosis can be made without exploration, no operation should be performed, inasmuch as it only shortens the patient’s life.” Although it is clear that gallbladder cancer has a tendency to spread early via lymphatic, hematogenous, and peritoneal metastases, it also has the unique ability to implant along biopsy tracts and wounds. Gallbladder cancer is often discovered incidentally after cholecystectomy performed for presumed benign disease; unfortunately, this operation is incomplete except for the earliest stage of disease.
Pessimistic attitudes about gallbladder cancer have persisted from its original description in 1778 (Kato et al, 1994) to more recent times and are supported by reports of overall 5-year survival of 5% and median survivals of less than 6 months. For advanced, untreated gallbladder cancer, the median survival is generally 2 to 5 months, and long-term survival is exceedingly rare (Perpetuo et al, 1978; Piehler & Crichlow, 1978). Systemic chemotherapy remains limited in its effectiveness, but with major improvements in systemic chemotherapy in other diseases, these benefits may begin to extend to patients with gallbladder cancer. The role of definitive resection, however, has now been shown to be effective in properly selected patients. Complete surgical removal of gallbladder cancer is the only potentially curative therapy. With improvements in imaging, staging, and hepatic and biliary resection, there is now hope for patients with nonmetastatic gallbladder cancer. Effective adjuvant therapy is lacking, however, and this represents a significant limitation of current treatment options.
Epidemiology
The incidence of gallbladder cancer varies by racial group, and it has been reported that internationally, incidence varies 20-fold based on geographic region (Randi et al, 2006). The highest incidence of gallbladder cancer is found in women living in India (21.5 cases per 100,000 population annually), Pakistan (13.8 per 100,000), and Ecuador (12.9 per 100,000). Approximately 9760 cases of gallbladder and other biliary cancers are diagnosed annually in the United States, with 3320 deaths annually (Jemal et al, 2010). In North America, high incidence rates are found among Native Americans and Hispanic women (Barakat et al, 2006). The annual incidence of gallbladder cancer in the United States is approximately 2 per 100,000 women and 1 per 100,000 men. In North America and Western Europe, urban areas and areas of lower socioeconomic status harbor higher rates of gallbladder cancer (Diehl, 1980; Zatonski et al, 1993). In these areas, limited access to health care delays and decreases the rate of cholecystectomy for gallstone disease (see Chapter 30) may lead to higher rates of gallbladder cancer (Serra et al, 1996). In the United States overall, gallbladder cancer is the most common cancer of the biliary tract and the fifth most common gastrointestinal cancer (Carriaga & Henson, 1995).
Across all populations that have been studied, women are approximately three times more likely to develop gallbladder cancer than are men (Lazcano-Ponce et al, 2001). This ratio is as high as 5 : 1 in countries such as Pakistan and Colombia (Randi et al, 2006). Although rare, reports of children (<21 years old) developing gallbladder cancer do exist (Rudolph & Cohen, 1972; De Aretxabala et al, 1994); it is generally a disease of advancing age, with the incidence steadily increasing to a plateau after age 60 years (Nakayama, 1991). Rare reports of familial gallbladder cancer exist but probably account for only a small percentage of cases (Trajber et al, 1982; Fernandez et al, 1994). Throughout the world, no consistent trend in the incidence of gallbladder cancer has been found, with decreases and increases reported in different countries. From 1978 to 1997, the incidence generally stabilized or declined in North America and Western Europe, but in Japan and South America, the incidence increased (Randi et al, 2006).
Obesity is now associated with increased death rates from many cancers. In a prospective cohort study of 900,000 U.S. adults, the relative risk of death from gallbladder cancer in women with a body mass index (BMI) of 30 to 34.9 was 2.13 compared with women with a BMI of 18.5 to 24.9. In men, the relative risk of death from gallbladder cancer with an elevated BMI was also significant at 1.76 (Calle et al, 2003). Other rare associations with gallbladder cancer include inflammatory bowel disease, primary sclerosing cholangitis (Lewis et al, 2007), and polyposis coli (Willson et al, 1987).
Etiology
The most consistently implicated etiologic factor in the development of gallbladder cancer is cholelithiasis and chronic inflammation. Of gallbladder cancer cases, 75% to 90% occur in the setting of cholelithiasis (Lazcano-Ponce et al, 2001; Serra & Diehl, 2002; Wanebo & Vezeridis, 1994). In one case-control study, the relative risk of gallbladder cancer was 10.1 in patients with stones larger than 3 cm (Diehl, 1983). Similar results were reported in another case-control study that compared gallstones in patients with gallbladder cancer with gallstones in patients with benign gallbladder disease (Roa et al, 2006). There were significantly more stones, heavier stones, and increased stone volume in the patients who had gallbladder cancer. The epidemiology of gallstones often parallels that of gallbladder cancer (Zatonski et al, 1997; Shrikhande et al, 2010); however, most patients with gallstones never have cancer develop, and a definitive cause-and-effect relationship has not been established. It is possible that stones and cancer share similar risk factors (see Chapter 8B), or stones may simply prompt a radiologic workup or cholecystectomy, increasing recognition in this group of patients.
There appears to be an association between cholesterol metabolism gene polymorphisms and a combined risk of gallbladder cancer and stones (Xu et al, 2010). Although nearly 90% of gallbladder cancer specimens contain stones, the incidence of gallbladder cancer in the population of patients with stones is 0.3% to 3%, which is low when considering this as the only risk factor. The other epidemiologic associations such as biliary-enteric fistulae, typhoid infection, and pancreaticobiliary maljunction also represent conditions in which the mucosa of the gallbladder is exposed to the effects of chronic inflammation. Although a true cause-and-effect relationship has never been proven, most epidemiologic data point to a significant relationship between chronic inflammation of the gallbladder and the development of neoplasia.
The presence of calcification in the wall of the gallbladder, otherwise known as porcelain gallbladder, is also a condition associated with a higher risk of gallbladder cancer. The calcification is probably the result of long-standing inflammation. The risk of malignancy within a porcelain gallbladder was previously reported to be extremely high (10% to 50%); modern series, however, have shown a much lower incidence (<10%) (Berk et al, 1973; Kim et al, 2009; Kwon et al, 2004; Stephen & Berger, 2001). The type of calcification seems to be associated with the degree of risk, with stippled calcification of the mucosa apparently representing a higher risk than diffuse intramural calcification.
Chronic inflammatory conditions of the gallbladder, such as cholecystoenteric fistula and chronic infection with typhoid bacillus, also have been associated with a risk for gallbladder cancer (Welton et al, 1979). Because bacterial colonization often accompanies chronic cholecystitis, bacteria has been proposed to play an important role in carcinogenesis. The argument against this etiology is that gallbladder cancer occurs in the setting of bacterial infection without stones and commonly occurs in the setting of stones without infection. The presence of an anomalous pancreaticobiliary junction with a long common channel between the pancreatic and bile duct also has been associated independently with gallbladder cancer risk and may be related to chronic inflammation (Chijiiwa et al, 1993). It has been difficult in experimental models, however, to induce gallbladder carcinoma with chronic inflammation. Fortner and Randall (1961) placed gallstones from patients with and without gallbladder carcinoma into the gallbladders of 126 cats. After 4 to 5 years, they found carcinoma in three cats, one of whose stones came from a patient without carcinoma. Despite these data, it is likely that chronic inflammation, regardless of cause, at the least predisposes to gallbladder carcinoma.
In patients with chronic inflammation as a predisposing factor, one hypothesis is that one or more carcinogenic exposures are required for carcinoma to develop. In numerous animal experiments, rates of gallbladder cancer were dramatically higher when inflammation was combined with known carcinogenic agents compared with exposure to the carcinogen alone (Enomoto et al, 1974; Kowalewski & Todd, 1971; Piehler & Crichlow, 1978). The composition of bile in patients with gallbladder cancer has been studied in attempts to identify carcinogenic agents. Higher concentrations of free radical oxidation products (Shukla et al, 1994) and secondary bile acids (Shukla et al, 1993) were found in gallbladders harboring cancer compared with gallbladders with gallstones alone. Some chemicals have been implicated in gallbladder carcinogenesis, including methyldopa (Broden & Bengtsson, 1980), oral contraceptives (Broden & Bengtsson, 1980), isoniazid (Lowenfels & Norman, 1978), and occupational exposure in the rubber industry (Mancuso & Brennan, 1970). None of these associations has been definitively proved (Pandey, 2006).
There is some suggestion of an adenoma-carcinoma progression in the development of gallbladder cancer. Severe dysplasia and carcinoma in situ are often adjacent to gallbladder carcinomas (Lazcano-Ponce et al, 2001). Unlike colorectal cancer, however, the association of polypoid gallbladder tumors and gallbladder cancer is rare. In fact, in patients with multiple gallbladder polyps, no increased risk of malignancy was found (Ito et al, 2009; Zielinski et al, 2009). Kubota and colleagues (1995) found that 11% of cholecystectomy specimens with gallbladder polyps had malignancies, and 88% of these polyps were larger than 1 cm. Yang and colleagues (1992) reported similar results with an 8% incidence of gallbladder cancer in gallbladder specimens with polyps, all more than 1 cm in size. Based on these studies, current practice recommendations are to perform cholecystectomy for gallbladder polyps larger than 1 cm.
Anatomic Considerations
It is important to understand the anatomy of the gallbladder, biliary tree, liver, and hepatic hilum to manage tumors of the gallbladder properly. The anatomy of the gallbladder is reviewed in Chapter 1A, Chapter 1B , but a few specific comments are worthy of review. The gallbladder is a partially intraperitoneal structure that lies attached to the undersurface of the liver on segments IVb and V. On the side of the gallbladder that is attached to the liver, there is no peritoneal covering; a fibrous lining known as the cystic plate occupies this space. When a simple cholecystectomy is performed, the plane between the muscularis of the gallbladder and the cystic plate is dissected, which is an inadequate resection for a malignancy involving this portion of the gallbladder. This fact makes a simple cholecystectomy inadequate for most gallbladder cancers. Because the body and fundus of the gallbladder generally lie at a distance from the major inflow structures to the liver, a limited segmental resection (segment IVb/V) is adequate to resect most tumors arising from this area of the gallbladder. The infundibulum and cystic duct encroach into the porta hepatis, however, and tumors of this area often involve the porta. The surgeon must be prepared to perform bile duct resections or major hepatic resections for tumors of the lower part of the gallbladder because major inflow structures are commonly involved.
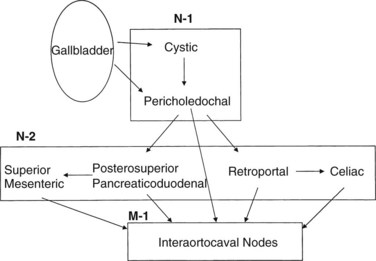
The lymphatic drainage of the gallbladder has been extensively studied (Shirai et al, 1992c); it is crucial to understand this drainage when radiologically or surgically staging patients with gallbladder cancer. The principal lymphatic drainage of the gallbladder as studied by dye injections is shown in Figure 49.1. When injected into the lymphatics of the gallbladder, the dye never ascends into the proximal porta hepatis lymphatics. The channels lead to the cystic and choledochal nodes first. From these nodes, the primary drainage areas are the retroportal and posterior-superior pancreaticoduodenal nodes. Progressing from these lower portal areas, the lymphatics course to the celiac, superior mesenteric, and interaortocaval nodes. An important finding was that some lymphatic material seemed to drain directly from the pericholedochal nodes to the interaortocaval nodes in the retropancreatic space. These connections may offer an explanation for the often advanced nature of gallbladder cancer at diagnosis and highlight the importance of analyzing the retropancreatic area radiologically and with a full Kocher maneuver at operation to stage patients with gallbladder cancer properly.
Pathology
Preneoplastic Lesions
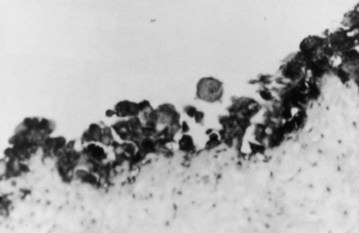
Similar to many gastrointestinal malignancies, progression from dysplasia to carcinoma in situ to frank invasive carcinoma is seen in gallbladder epithelium (Albores-Saavedra et al, 1986; Kozuka et al, 1982). When surrounding mucosa is sectioned in cases of gallbladder cancer, more than 90% of the cases harbor high-grade dysplasia or carcinoma in situ. Although chronic inflammation of the gallbladder can cause cellular atypia, it is generally easy to differentiate this from dysplasia and carcinoma in situ (Fig. 49.2). Carcinoma in situ may appear in the Rokitansky-Aschoff sinuses and may be mistaken for invasive carcinoma. Although it is impossible to know definitively the time course between dysplastic changes and invasive carcinoma, estimations have been made. In one series, the rate of progression of precursor lesions to invasive carcinoma was estimated to be approximately 15 years based on the mean ages of patients at each stage (Roa et al, 1996). Another study found a 5-year difference between the mean ages of patients with dysplasia and those with carcinoma in situ, and a 10-year difference was found between the mean ages of patients with carcinoma in situ and those with invasive carcinoma (Albores-Saavedra et al, 1986).
The precancerous nature of gallbladder polyps is more controversial; however, it provides some evidence that an adenoma-adenocarcinoma progression exists. Gallbladder polyps have been noted in 3% to 6% of patients undergoing ultrasonography (US). Most are cholesterol polyps with no malignant potential (Choi et al, 2008; Zielinski et al, 2009). The incidence of carcinoma in nonadenomatous polyps—cholesterol polyps, inflammatory polyps, and hyperplastic polyps—is close to zero (Canturk et al, 2007; Choi et al, 2008; Ito et al, 2009). Approximately 1% of cholecystectomy specimens contain adenomatous polyps (Aldridge & Bismuth, 1990; Fong & Malhotra, 2001). In another series of 123 gallbladder polyps, 21 (17%) were found to be adenomatous, and seven (6%) were found to harbor malignancy. Risk of malignancy was associated with increasing age, size greater than 1 cm, and the presence of a single polyp (Yeh et al, 2001). Ito and colleagues (2009) reviewed 417 patients with gallbladder polyps found on US. Only 7% of patients had polyps larger than 1 cm. Among the 80 patients who underwent cholecystectomy, neoplastic (adenomatous) polyps were found in 10%, and one patient had a carcinoma in situ (in a 14-mm polyp). No patient was found to harbor an invasive malignancy.
Kozuka and colleagues (1982) described seven adenomas showing histologic progression into malignancy and showed that 19% of invasive carcinomas had adenomatous components. In a series of 182 resected gallbladder polyps, 13 (7%) were found to harbor a malignancy, which was related to the size of the polyp (Yang et al, 1992). One report described a higher incidence of malignancy in gallbladder polyps arising in the face of primary sclerosing cholangitis (Buckles et al, 2002).
Gross Morphology
Early-stage gallbladder cancers are difficult to distinguish from the findings typical of chronic cholecystitis because they both often present as a thickened gallbladder wall in the face of inflammation. This similar presentation accounts for the frequent discovery of gallbladder cancer incidentally in a cholecystectomy specimen. The overall incidence of gallbladder cancer discovered during cholecystectomy for presumed benign disease is less than 1% (Bazoua et al, 2007). As gallbladder tumors progress, they can present in numerous ways. The gallbladder may become distended with tumor, or it may become contracted and collapsed. Approximately 60% of tumors originate from the fundus, 30% from the body, and 10% from the neck of the gallbladder (Albores-Saavedra et al, 1986; Daines et al, 2004; Rajagopalan et al, 2004). Tumors in the lower end of the gallbladder may obstruct the neck or cystic duct, leading to hydrops. Advanced tumors of the neck, infundibulum, or cystic duct may infiltrate the porta hepatis and result in major vascular invasion, jaundice, and even hepatic atrophy. This presentation is often indistinguishable from a hilar cholangiocarcinoma.
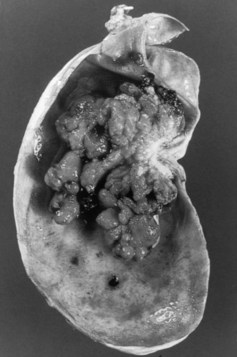
Gross descriptions of gallbladder cancer have been grouped into infiltrative, nodular, combined nodular-infiltrative, papillary, and combined papillary-infiltrative forms (Albores-Saavedra et al, 2005; Sumiyoshi et al, 1991). Most tumors have an infiltrative pattern as part of their presentation, which causes thickening and induration of the gallbladder wall (Fig 49.3). These types of tumors seem to spread in a subserosal plane and can invade the whole gallbladder wall and even invade into the porta hepatis, causing jaundice from diffuse biliary involvement. These types of tumor can also diffusely invade the liver. Nodular types of tumors tend to grow as a more circumscribed mass. Despite invasion into the liver, these lesions are more easily resected because they are less diffuse in their invasive pattern. Papillary tumors of the gallbladder are polypoid lesions with frondlike extensions that give a cauliflower-like appearance (Fig. 49.4). Despite the fact that papillary tumors can grow quite large, they tend to have a better prognosis than the other gross types; this is likely related to the less invasive nature of these tumors. Even at large sizes, papillary gallbladder tumors may have minimal invasion into the gallbladder wall (Albores-Saavedra et al, 2005).
Histology (See Chapter 47)
Table 49.1 shows the classification of malignant gallbladder tumors, and Table 49.2 summarizes the relative incidence of the various histologic types of gallbladder cancer. Over a 10-year period at Memorial Sloan-Kettering Cancer Center (MSKCC), 391 (90%) of the 435 patients with gallbladder cancer had adenocarcinomas. Additional diagnoses were squamous/adenosquamous carcinoma (4%), neuroendocrine carcinoma (3%), sarcoma/adenosarcoma (1.6%), unspecified carcinoma (1.1%), and melanoma (<1%) (Duffy et al, 2008). Most gallbladder tumors can be characterized histologically, but more than one histologic pattern is commonly found in each tumor. The only histologic subtype that seems to have prognostic significance is the papillary tumor. Papillary adenocarcinomas of the gallbladder tend to have a better prognosis than the other subtypes (Albores-Saavedra et al, 2005; Carriaga & Henson, 1995). This improved prognosis is likely related to the tendency of this tumor to be noninvasive or minimally invasive. When this tumor becomes invasive, however, it can metastasize and have a prognosis typical of the pathologic stage (Albores-Saavedra et al, 2005).
Table 49.1 Classification of Malignant Tumors of the Gallbladder
| Epithelial Tumors |
| Mesenchymal Tumors |
| Other Tumors |
Modfied from Albores-Saavedra J, Henson DE, 1986: Tumors of the gallbladder and extrahepatic bile ducts. In Atlas of Tumor Pathology, Second Series. Bethesda, MD, Armed Forces Institute of Pathology.
Table 49.2 Relative Incidence of Gallbladder Cancer by Histologic Type
| RELATIVE INCIDENCE (%) | ||
|---|---|---|
| Histologic Type | Carriaga & Henson, 1995 | Duffy et al, 2008 |
| Carcinoma | 99 | 94 |
| Adenocarcinoma | 89.4 | 90 |
| Papillary | 5.7 | — |
| Mucinous and mucin producing | 5.3 | — |
| Squamous cell | 1.8 | 4 |
| Other and unspecified | 7.8 | 1.4 |
| Sarcoma | 0.2 | 1.6 |
| Neuroendocrine | — | 3 |
From Carriaga MT, Henson DE, 1995: Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer 75(Suppl 1):171-190; and Duffy A, et al, 2008: Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Center (MSKCC). J Surg Oncol 98(7):485-489.
Throughout the medical literature are many case reports and small case series of other rare gallbladder cancers. Primary sarcomas of the gallbladder—including embryonal rhabdomyosarcoma, leiomyosarcoma, malignant fibrous histiocytoma, angiosarcoma, and Kaposi sarcoma—have been reported and are extremely rare. Other reported uncommon gallbladder cancers include carcinosarcoma, carcinoid, lymphoma, melanoma, and metastatic tumors to the gallbladder. Although reports have suggested a poorer outcome for oat-cell carcinomas (Henson et al, 1992) and adenosquamous carcinomas (Yamaguchi & Enjoji, 1988), these conclusions are based on small numbers, and comparisons of such rare tumors are impossible.
Gallbladder cancers also have been grouped into metaplastic and nonmetaplastic types based on metaplastic changes in the tumors; this is similar to the classification of gastric cancers into intestinal and diffuse types. The two predominant types of metaplasia that are precursors to malignancy in the gallbladder mucosa are pseudopyloric and intestinal (Duarte et al, 1993). The intestinal type has a higher rate of carcinoma. The progression from metaplasia to carcinoma in situ to carcinoma is evident in that patients with invasive cancer are 15 and 5 years older than patients with dysplasia and carcinoma in situ, respectively (Roa et al, 1996).
Histologically, gallbladder cancers are graded into four categories that range from well differentiated to poorly differentiated. This differentiation does not have a significant impact on prognosis except for the observation that most papillary tumors are well differentiated, which may account for the better prognosis seen in these types of tumors. Most gallbladder cancers are poorly differentiated at presentation. DNA ploidy has been studied in gallbladder tumors and corresponds to the histologic grade but does not correlate with prognosis (Baretton et al, 1994). Although it is clear that gallbladder cancers can be differentiated based on histologic type and grade, the most important prognostic factor is the stage at presentation, making grade and type prognostically unimportant.
Molecular Biology (See Chapter 8B)
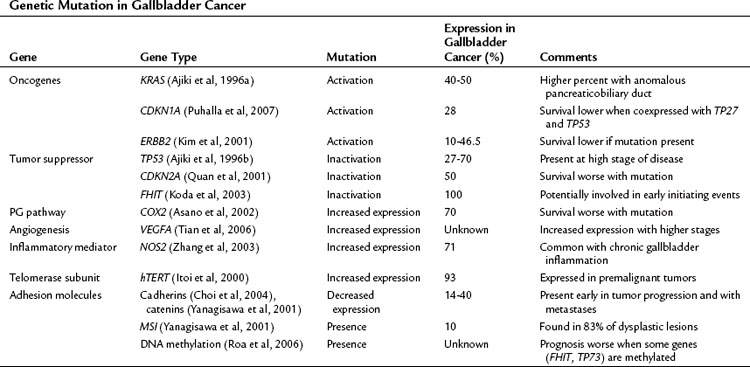
The precise pathway linking different genetic mutations responsible for gallbladder cancer is not clear; however, multiple genetic defects are known to be associated with gallbladder cancer (Lazcano-Ponce et al, 2001; Rashid, 2002; Sasatomi et al, 2000). Tumor suppressor genes, oncogenes, microsatellite instability, and DNA repair genes all participate in the initiation of gallbladder cancer (Table 49.3) (Goldin & Roa, 2009). Several of these genetic mutations have been identified in case-control studies and have been combined into risk scores. For example, Srivastava and colleagues (2010) compared genetic variations in three DNA repair genes—ERCC2, MSH2, and OGG1—among 230 patients with gallbladder cancer and 230 control subjects. They found that patients with more than six of the variant alleles that were studied had a fourfold increased risk for developing gallbladder cancer.
Serologic biomarkers can potentially be useful to identify groups of patients at increased risk for gallbladder cancer. When used together, polymorphisms CA242 and CA125 have been associated with high sensitivity and specificity for differentiating between cholelithiasis and gallbladder cancer (Shukla et al, 2006). Several other serum markers may identify patients at high risk for developing gallbladder cancer, regardless of the presence of cholelithiasis. These markers include the NAT2 slow acetylator phenotype, the X(+), D haplotype of apolipoprotein B, and the D allele of lipoprotein receptor–associated protein (LRPAP1) insertion/deletion polymorphism (Pandey, 2006; Pandey et al, 2007a, 2007b).
Pattern of Spread
An autopsy study showed a 94% incidence of lymphatic metastases and a 65% incidence of hematogenous dissemination (Kimura et al, 1989); however, autopsy studies represent the end stage of disease, with sufficient time for extensive metastases to develop. It has been postulated that hematogenous metastases originate from small veins extending directly from the gallbladder into the portal venous system of the gallbladder fossa. These connections can lead to segments IV and V of the liver or via larger veins to the portal venous branches of segments V and VIII (Boerma, 1994). A review of the incidence of regional invasion and metastases at the time of diagnosis and treatment is summarized in Table 49.4.
Table 49.4 Incidence of Regional Invasion and Metastasis at the Time of Diagnosis and Treatment Based on a Literature Review
| Pathologic Finding | Relative Incidence (%) |
|---|---|
| Confined to gallbladder wall | 10 |
| Liver invasion | 59 |
| Common bile duct infiltration | 35 |
| Lymphatic invasion and regional lymphatic metastases | 45 |
| Gallbladder vein infiltration | 39 |
| Portal vein or hepatic artery invasion | 15 |
| Adjacent organ invasion (excluding liver) | 40 |
| Perineural invasion | 42 |
| Liver metastasis | 34 |
| Distant metastasis (excluding liver) | 20 |
Data from Boerma EJ, 1994: Towards an oncological resection of gall bladder cancer. Eur J Surg Oncol 20(5):537-544.
It is also important to understand the patterns of spread after complete resection because these facts can help guide approaches to adjuvant therapy. Autopsy studies have shown widely disseminated disease, including liver metastases, in greater than 90%; abdominal lymph node metastases in greater than 80%; and peritoneal metastases in 60% of patients (Perpetuo et al, 1978). The only common extraabdominal site of distant metastases is the lung, but lung metastases are rarely seen in the absence of advanced intraabdominal disease. In an attempt to define sites of first recurrence after complete resection, Jarnagin and colleagues (2003) found that only 15% of patients had locoregional recurrence as the only site of recurrence, and most (85%) had recurrence involving a distant site. This finding shows the minimal potential utility of adjuvant locoregional strategies and underscores the importance of effective adjuvant systemic therapies.
Clinical Presentation
Three clinical scenarios are common for gallbladder cancer: 1) final pathology after routine cholecystectomy identifies gallbladder cancer; 2) gallbladder cancer is discovered intraoperatively; and 3) gallbladder cancer is suspected before surgery (Miller & Jarnagin, 2008). The clinical presentation varies depending on geographic location, rates of gallbladder cancer, and referral patterns. In a study of 435 patients with gallbladder cancer treated at MSKCC during a 10-year period, 47% had cancers discovered incidentally at the time of routine laparoscopic cholecystectomy (Duffy et al, 2008), and 53% were initially seen with advanced (16%) or disseminated (37%) disease. Given the high percentage of patients diagnosed with gallbladder cancer after routine cholecystectomy for presumed benign gallbladder disease, it is appropriate for the surgeon to inspect the gallbladder mucosa at the completion of the resection if there are any concerns. Frozen-section analysis should be used to examine any suspicious areas.
Gallbladder cancer is notorious for being asymptomatic in its early stages. When symptoms do occur, however, gallbladder cancer tends to present in a similar manner to biliary colic or chronic cholecystitis. Careful history taking often shows a history of constant right upper quadrant pain rather than the typical episodic crampy pain of biliary colic. The diagnosis of gallbladder cancer should be considered in an elderly patient with constant right upper quadrant pain with weight loss, anorexia, or both because weight loss, anorexia, and jaundice in particular are signs of advanced disease (Duffy et al, 2008). The presence of a palpable mass is also an ominous finding that predicts a high rate of unresectability and advanced disease (Thorbjarnarson & Glenn, 1959). The presence of jaundice is an especially ominous finding. In a report by Hawkins and colleagues (2004), 82 (34%) of 240 patients presented with jaundice. Of these 82 patients, only six (7%) were resectable, and all had recurrence or died of disease within 2 years. The median survival in jaundiced patients was 6 months compared with 16 months in patients presenting without jaundice.
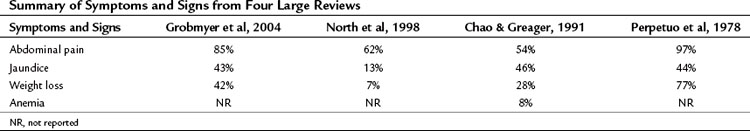
The incidence of symptoms and signs at presentation is summarized in Table 49.5. A report from New York Hospital reviewed their experience with gallbladder cancer from 1915 through 2000 (Grobmyer et al, 2004). Throughout the years, the presentation was remarkably similar in that most patients had advanced disease at presentation. Over time, however, a decrease was reported in the percentage of patients who presented in the more recent era with weight loss (95% vs. 42%), palpable mass (50% vs. 9%), and nausea/vomiting (97% vs. 70%). The percentage of patients presenting with abdominal pain and jaundice was similar (approximate 85% and 50%, respectively).
Laboratory examination generally is not helpful except to identify the typical signs of advanced disease, such as anemia, hypoalbuminemia, leukocytosis, and elevated alkaline phosphatase or bilirubin levels (Grobmyer et al, 2004; Thorbjarnarson & Glenn, 1959). The only tumor markers studied that are of any potential value are carcinoembryonic antigen (CEA) and carbonin anhydrase 19-9 (CA19-9). An elevated CEA tends to be specific for gallbladder cancer (90%), but it lacks sensitivity (50%) when used as a screening test in cancer patients compared with patients who have benign gallbladder diseases (Strom et al, 1990). CA19-9 is more consistent as a marker for gallbladder cancer, with sensitivities and specificities of approximately 75% at a level greater than 20 U/mL (Ritts et al, 1994). Overall, serum tumor markers are of minimal clinical value compared with clinical awareness, a heightened level of suspicion in appropriate cases, and good-quality imaging studies. As is typical of the utility of serum tumor markers, they can be helpful in following a patient for recurrence, if they are elevated before treatment and normalize after treatment.
Radiologic Investigation
In the era before the availability of routine real-time US and computed tomography (CT), gallbladder cancer was rarely diagnosed preoperatively. With the development of rapid and more widely available imaging modalities, patients who have suspected or incidentally diagnosed gallbladder cancer should have high-resolution cross-sectional imaging for adequate staging. Because most patients present with advanced disease, it is important to try to establish the diagnosis and extent of disease with imaging to minimize the number of patients who have to undergo a nontherapeutic surgical exploration. Except for the earliest stage of disease, this should now be possible in most patients. In addition to the modalities available for examining the gallbladder and liver, chest radiographs or CT scan should be obtained during the complete staging workup to rule out pulmonary metastases. It is rare, however, to find pulmonary metastases without locally advanced or intraabdominal metastatic disease (Lee et al, 2010).
US is an excellent imaging modality for the gallbladder. Findings such as discontinuous mucosa, echogenic mucosa, and submucosal echolucency are more common in early malignancy compared with benign gallbladder disease. Doppler assessment of blood flow through areas of mucosal abnormalities can help to differentiate early malignancy from benign disease, and newer contrast-enhanced US techniques may improve detection confidence even further (Sato et al, 2001). In one study, a polypoid mass was present in 27% of cases, and a gallbladder-replacing or invasive mass was present 50% of the time (Wibbenmeyer et al, 1995). US is a good modality for evaluating the direct extension of gallbladder cancer. One retrospective study reported that in 203 patients with gallbladder cancer, a mass was identified in 177 patients (87%) on preoperative US (Pandey et al, 2000). US was limited, however, in identifying lymph node metastases in pericholedocal and peripancreatic nodes. Because most cases are advanced, the most typical findings in gallbladder cancer are an inhomogeneous mass replacing all or part of the gallbladder (Bach et al, 1998; Franquet et al, 1991). Diffuse thickening of the gallbladder wall also is a common finding on cross-sectional imaging and on US, but it can be difficult to differentiate from benign inflammatory changes.
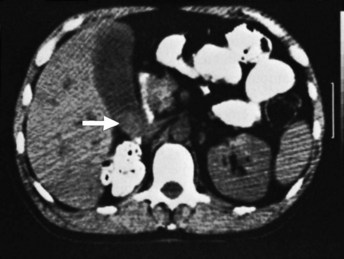
Cross-sectional imaging with CT or magnetic resonance imaging (MRI) is an important part of the preoperative assessment of gallbladder cancer (see Chapters 16 and 17). These techniques provide crucial information about the local extent of disease and show whether distant metastases are present. The most common finding on CT is a mass involving all or part of the gallbladder. Extension into local organs, particularly the liver, usually can be discerned. In one study of patients with gallbladder masses, asymmetric wall thickening was found in 45% of patients, a mass replacing the gallbladder was found in 35%, and an intraluminal mass was found in 20% (Fig. 49.5) (Kalra et al, 2006). Assessment of regional and distant lymph nodes is important and can be done with CT. With criteria of size greater than 1 cm and a ringlike heterogenous enhancement, accuracy rates greater than 80% have been reported (Ohtani et al, 1993). Although large lymph nodes replaced with tumor are relatively easy to identify with CT, false-negative examination results continue to be a problem because many involved lymph nodes can be small, with minimal tumor. CT is 71% to 84% accurate in staging gallbladder cancer. In one study of 118 patients with gallbladder cancer, CT was 79% accurate for differentiating T1 versus T2 tumors, 93% accurate for differentiating T2 versus T3 tumors, and 100% accurate for differentiating T3 versus T4 tumors (Kim et al, 2008). The overall accuracy improved from 72% to 85% when multiplanar reconstructions were added to conventional axial imaging.
The utility of MRI for evaluating patients with gallbladder cancer has increased in recent years. Improvements in MRI technology over the last 2 decades has been dramatic, with wider availability of MRI cholangiography and angiography. Invasive diagnostic cholangiography has largely been replaced by MRI cholangiography in most high-volume centers (Schwartz et al, 2002). Likewise, the use of diagnostic angiography has been replaced by CT/MRI angiography, and such modern equipment can provide detailed imaging of the related vessels at the hepatic hilum. Analyses of MRI for the assessment of gallbladder cancer have shown sensitivities of 70% to 100% for hepatic invasion and 60% to 75% for lymph node metastases (Kim et al, 2002; Schwartz et al, 2002). It is unclear whether MRI adds to the results obtained from CT scan. In one study of 25 patients with gallbladder cancer, MRI/MRCP did not change the preoperative stage as determined by CT scan (Rao et al, 2005).
The development and use of fluorodeoxyglucose (FDG) positron-emission tomography (PET) has resulted in superior staging and diagnosis of many tumors. Most gallbladder cancers are visible on PET scan; theoretically, PET imaging could help differentiate between benign and malignant tumors and can help diagnose extrahepatic spread (Petrowsky et al, 2006). PET, however, is limited in differentiating between benign inflammatory states, such as postcholecystectomy, and malignancy (Corvera et al, 2008). PET appears to be more accurate in diagnosing metastatic disease than is CT scan. In a study of 61 patients with biliary tract malignancies, PET/CT had a sensitivity of 100% compared with 25% for CT alone (P < .001), and PET alone changed surgical management in 17% of cases (Petrowsky et al, 2006
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree