Chapter 50C Cancer of the bile ducts
Perihilar cholangiocarcinoma with emphasis on presurgical management
Overview
Perihilar cholangiocarcinoma is often diagnosed as advanced disease at initial presentation and is difficult to treat (de Groen et al, 1999). Hepatobiliary resection represents the definitive surgery and most effective treatment for this disease (Bismuth et al, 1992; Blumgart et al, 1984; Burke et al, 1998; Hemming et al, 2005; Kawasaki et al, 2003). Combined portal vein resection (Ebata et al, 2003; Nimura et al, 1991a), combined hepatic artery resection, or hepatopancreatoduodenectomy (Ebata et al, 2007; Nimura et al, 1991b) have also been aggressively used at leading hepatobiliary centers in Japan, Korea, Europe, and the United States (Igami et al, 2010; Lee et al, 2010; Miyazaki et al, 2010; Rocha et al, 2010; Unno et al, 2010; Young et al, 2010). However, hepatobiliary resection for perihilar cholangiocarcinoma is well known to result in higher morbidity or mortality rates than those for simple hepatectomy because of impaired liver function with cholestasis or cholangitis (Belghiti et al, 2000; Nagino et al, 1993, 2001). To eradicate the involvement of the hilar duct, extensive hepatectomy should be designed based on tumor extension and individual liver functional reserve. It is of particular importance that the presurgical management is optimized. Perioperative management and surgical techniques have evolved over the past 30 years, which has succeeded in reducing postoperative complications. Current methods and recommendations for the presurgical management of perihilar cholangiocarcinoma are outlined in this section.
Basic Anatomic Pathology Dictates Surgical Strategy (See Chapter 47)
According to the Johns Hopkins classification for cholangiocarcinomas (Nakeeb et al, 1996), perihilar cholangiocarcinomas are defined as cholangiocarcinomas involving or requiring resection of the hepatic duct conference. Because the boundary between the intrahepatic and extrahepatic bile duct is unclear, perihilar tumors are potentially composed of two types of tumors: extrahepatic hilar cholangiocarcinomas, which arise from the large hilar bile duct, and intrahepatic hilar cholangiocarcinomas, which have an intrahepatic component and involve the hepatic hilum. These two tumors exhibit the same clinical presentation, demonstrate similar radiologic features, require an identical surgical intervention, and have comparable histologic characteristics (Ebata et al, 2009). In addition, they can be well classified by the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging for extrahepatic bile duct cancer (Greene et al, 2002), providing well-stratified survival (Ebata et al, 2009). Thus these two tumor categories overlap considerably, as already suggested by Klatskin (1965); therefore both tumors should be treated under the banner of perihilar cholangiocarcinoma (Ebata et al, 2009; Nakeeb et al, 1996).
Cholangiocarcinoma microscopically infiltrates the surrounding tissue beyond the macroscopic tumor extent (main tumor) (Ebata et al, 2002; Sakamoto et al, 1998). The lateral extension of invasive cancer is limited within 1 cm in both directions beyond the main mass in most tumors, and noninvasive cancer extension is limited within 2 cm in 90% of tumors (Ebata et al, 2002). These observations indicate that a 1-cm margin and a 2-cm margin of the duct are recommended for eradication of invasive and noninvasive cancer cells, respectively.
However, in perihilar cholangiocarcinomas, it is often difficult to obtain a satisfactory margin length that meets the above requirements because of the anatomy of the liver, especially in the proximal direction. Therefore a proximal resection line is set at the farthest proximal point as technically possible, and pathologic assessment with frozen sections of the ductal stump is widely used to confirm a negative ductal margin. Flaws in the results of intraoperative frozen section have been reported, including 1) the diagnostic difficulty of differentiation between intraepithelial neoplasm and reactive/regenerative epithelial changes, 2) the absence of a standard histologic classification (Konishi et al, 2008), 3) difficulty in obtaining sufficient material for diagnosis because of crushing and exfoliation resulting from manipulation (Konishi et al, 2008), and 4) relatively low diagnostic accuracy, ranging from 25% to 57% (Okazaki et al, 2002; Yamaguchi et al, 2005). These limitations suggest an inherent limitation of frozen-section examination. When the ductal margin is positive by intraoperative frozen-section diagnosis, several factors must be taken into account. First, prognostic indicators after surgery are nodal metastasis, poor histologic grade, portal vein invasion, and positive surgical margins (Ebata et al, 2003, 2009). Of these predictors, nodal involvement is observed most frequently, in more than 50% of patients (Ebata et al, 2003; Igami et al, 2009; Kitagawa et al, 2001). Second, a positive ductal margin with noninvasive cancer, unlike invasive cancer, may not have a negative impact on survival (Igami et al, 2009; Nakanishi et al, 2010; Sasaki et al, 2007; Wakai et al, 2005). It has been noted that residual in situ carcinoma does not result in early fatal recurrence; however, over time, it steadily and insidiously progresses to an anastomotic tumor mass (Nakanishi et al, 2010). Third, additional resection of more than 5 mm of the proximal duct is difficult, practically, after maximal or near-maximal resection of the duct. Such limited resection of a margin-positive proximal duct does not improve survival, even when a negative margin can be achieved after additional resection (Shingu et al, 2010). Overall, the clinical value of additional resection of the proximal duct in perihilar cholangiocarcinoma is limited.
A right hemihepatectomy or trisectionectomy with en bloc resection of the caudate lobe is generally recommended for perihilar cholangiocarcinomas (Kawasaki et al, 1994; Neuhaus et al, 1999). This right-sided resection satisfies the nontouch technique, en bloc resection, and wide tumor-free margins and consequently leads to good local control and a favorable prognosis (Jonas et al, 2008). However, the optimal procedure (extensive vs. limited surgery) for Bismuth type I and II tumors is still under debate. Tumor configuration is classified into papillary, nodular, or diffusely infiltrating tumors (Henson et al, 2000). Papillary tumors display less invasive behavior and, accordingly, they have a more favorable survival than other tumor types (Albores-Saavedra et al, 2000; Jarnagin et al, 2005). Recently, the surgical strategy for Bismuth type I and II tumors were reappraised based on gross tumor type (Ikeyama et al, 2007). When optimizing by the gross tumor configuration, right-sided hepatectomy is suitable for nodular or flat tumors that display an infiltrating nature in the advancing margin; whereas a limited surgery—including resection of S1, S1+4, or bile duct resection alone—is acceptable for papillary tumors, provided the surgical margin is negative (Ikeyama et al, 2007).
In addition, there is a specific tumor type called superficial spreading type cholangiocarcinoma (Igami et al, 2009; Nakanishi et al, 2008; Sakamoto et al, 1998), which is defined as cholangiocarcinoma with superficial spread; that is, a noninvasive cancer extension longer than 2 cm. The mean length of the superficial spread is 54 mm, suggesting that this tumor often requires extensive resection to obtain a negative ductal margin. In contrast, superficial spreading-type cholangiocarcinomas are also characterized by less advanced pT and pN classifications and a more differentiated histologic grade, which leads to a favorable prognosis (Igami et al, 2009; Nakanishi et al, 2008). In such patients, complete eradication of the superficial spreading lesion (in situ carcinoma) is necessary. Taken together, these findings suggest that a patient with this specific tumor may benefit from extensive surgery, such as hepatopancreatoduodenectomy (Ebata et al, 2007; Nimura et al, 1991b).
Hepatobiliary surgeons should keep the possibility of multiple synchronous cancers in mind. Multiple tumors are more common than previously thought: the incidence ranges from 5% to 9% (Gertsch et al, 1990; Kozuka et al, 1984; Kurosaki et al, 1997). Thus, multiplicity is a feature of biliary neoplasia that may be clinically relevant in some patients. Gertsch and colleagues (1990) proposed three criteria for multiplicity: 1) no direct continuity between the two tumors, 2) a growth pattern typical of a primary tumor, and 3) clear histologic differences between the two tumors. However, a true synchronous tumor and metastatic tumor originating from a biliary site elsewhere cannot always be clinically distinguished. Interestingly, a second tumor may not be identified by the preoperative radiologic workup but rather by pathologic assessment. Most second biliary tumors are an incidental early stage gallbladder cancer (Gertsch et al, 1990; Kurosaki et al, 1997). This indicates a need for intraoperative surveillance, meticulous inspection of the specimen immediately after resection, and appropriate sampling of the tissue for pathology.
The concept of field cancerization potentially explains the multiplicity of biliary tumors. Recently, pathoepidemiologic evidence for multiple biliary tumors has been reported (Henson et al, 2009). The age-specific incidence of biliary tract and gallbladder tumors in the United States demonstrates a parallel incline pattern, indicating that the rate of cancer development is identical and that the sequential molecular/cellular events toward the carcinogenesis among multiple isolated lesions share common pathways.
Biliary Drainage In The Preoperative Setting (See Chapter 7, Chapter 18 )
It is well known that chronic obstruction of the bile duct causes hepatic fibrosis, secondary biliary cirrhosis, and portal hypertension. When biliary obstruction is appropriately relieved, the fibrosis and portal hypertension can be ameliorated (Hammel et al, 2001). Furthermore, prolonged obstruction often induces acute obstructive suppurative cholangitis (Glenn & Moody, 1961), which can only be treated by biliary drainage (Berliner & Burson, 1973). The purposes of biliary drainage are summarized as follows: 1) treatment of biliary sepsis (Kanai et al, 1996), 2) relieving jaundice and recovery of functional capacity, 3) diagnosis of lateral tumor extension (Nimura et al, 1989), 4) liver function assessment using bile samples (Higuchi et al, 2005; Kurumiya et al, 2003; Maeda et al, 1999; Takeuchi et al, 1997; Uesaka et al, 1996), and 5) improvement of poor food intake (Padillo et al, 2001).
Obstructive jaundice associated with perihilar cholangiocarcinoma differs from jaundice associated with distal tumors. In the latter, a single catheter is enough for biliary decompression of the entire liver; in the former, multiple drainage catheters are necessary because perihilar cholangiocarcinomas frequently isolate the intrahepatic biliary tree into several subunits (Klatskin, 1965). Under such difficult conditions, it remains controversial which method is better: an endoscopic approach versus a percutaneous approach or unilateral versus bilateral drainage. Several authors (Gholson & Burton, 1991; Polydorou et al, 1991; Speer et al, 1987) have stressed the advantages of endoscopic retrograde biliary drainage (ERBD) because it is associated with low procedure-related morbidity and mortality rates, a shorter hospital stay, and good quality of life (external tube free). However, ERBD is associated with a high incidence of cholangitis because of catheter occlusion and/or inability to perform multiple drainages (Andersen et al, 1989; Chang et al, 1998; Liu et al, 1998; Rerknimitr et al, 2004). Hence, percutaneous transhepatic biliary drainage (PTBD), rather than ERBD, may be indicated for perihilar cholangiocarcinomas (Nomura et al, 1997).
Recently, endoscopic nasobiliary drainage (ENBD) has been recommended as a preoperative drainage procedure for perihilar cholangiocarcinomas (Arakura et al, 2009; Maguchi et al, 2007). Arakura and colleagues (2009) reported that a single ENBD was enough to relieve jaundice in 46 of 62 patients (74%) with perihilar cholangiocarcinoma, and additional PTBD was necessary in only eight patients. Early complications, such as acute cholangitis, dislodgement, and hemobilia, occur 14.5%, 0%, and 0% of the time, respectively, indicating the low morbidity associated with ENBD. Furthermore, ENBD carries no risk of seeding metastases along the catheter tract, which could compromise the results of curative resection (Fig. 50C.1). In our experience, seeding metastases are encountered in up to 5% of patients with cholangiocarcinoma who underwent resection after PTBD.
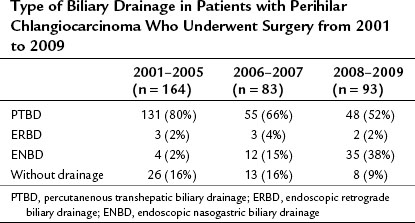
Obstructive jaundice appears when more than 75% of the liver is involved (Abraham et al, 2002), and based on the experience of palliative biliary drainage, it is usually relieved by drainage of a single liver segment (Abraham et al, 2002; Ballinger et al, 1994; Van Laethem et al, 2003). In addition, unilateral drainage promotes hypertrophy of the future liver remnant (FLR), which leads to a good functional reserve (Ishizawa et al, 2007). Thus the current strategy of preoperative biliary drainage for perihilar cholangiocarcinoma is summarized as follows: first, all ducts in the FLR should be drained; second, ENBD is superior to PTBD. Previously, we preferred PTBD (Nagino et al, 1992; Nimura, 1993; Nimura et al, 1995, 1998) but have not changed the policy for preoperative drainage since 2007. The first-line treatment is currently a single ENBD placed in the FLR alone, irrespective of the Bismuth tumor type. PTBD is performed as a second-line procedure only when 1) multiple, separated ducts are present in the FLR; 2) total liver drainage is necessary for prolonged cholestasis or segmental cholangitis of the undrained lobe; or 3) the ENBD tube is dislodged or kinked. As a result, the proportion of patients treated with ENBD has recently increased, but PTBD has decreased, although it is still needed for approximately half of the patients (Table 50C.1).
Table 50C.1 Type of Biliary Drainage in Patients with Perihilar Chlangiocarcinoma Who Underwent Surgery from 2001 to 2009
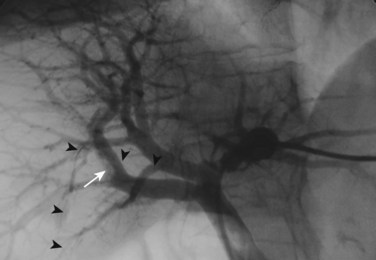
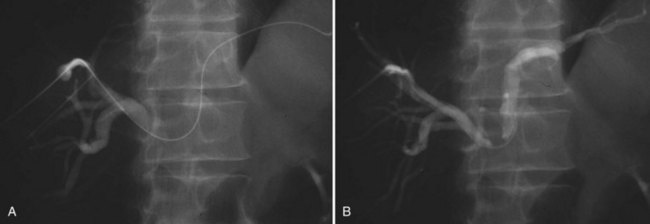
The current role of PTBD is mainly to salvage the procedure if ENBD fails. In this difficult scenario, multiple PTBD is frequently required to decompress the whole liver, except for the caudate lobe (Nagino et al, 1992; Nimura et al, 1995). Our favorite method for PTBD is Takada’s direct anterior approach (Takada et al, 1976), using a Takada needle, approximately 3 mm in diameter, which is composed of an internal metallic needle and an outer Teflon sheath (Fig. 50C.2). First, the target duct is opacified with a fine needle; next, the needle is advanced parallel to the radiograph into the duct; and finally, a 6-Fr soft catheter is installed through the sheath. Previously, the intrahepatic bile duct of the FLR was directly punctured; however, vascular injury is possible with such an approach (Fig. 50C.3). A large series of PTBD between 1991 and 2006 (Dowsett, 1989; Fidelman et al, 2008) showed that the risk of procedure-related arterial injury is 2.2%, and the incidence has not changed since the 1970s and 1980s. To prevent accidental vascular injury, the ipsilateral approach, similar to portal vein embolization (Nagino et al, 1996), is recommended when it is possible. In this approach, the bile duct in the sector to be removed is punctured, and the straight single catheter is introduced into the duct in the FLR via the hilar duct, which is stenotic as a result of tumor. Although this procedure requires a meticulous searching technique using a flexible guidewire, it offers complete bilateral drainage with the least numbers of single catheters (Fig. 50C.4). PTBD with the ipsilateral approach should be considered when total liver drainage is necessary.
Although biliary drainage is the routine preoperative management strategy for jaundiced patients in Japan (Nagino et al, 2008), randomized control trials (RCTs) conducted in Western countries have raised questions about the effectiveness of preoperative biliary drainage (see Chapters 7 and 50B). The results of these trials showed no significant difference in postoperative morbidity and mortality among patients who received preoperative biliary drainage and those who did not. These RCTs concluded that, considering the potential risk related to PTBD, preoperative biliary drainage has no advantage (Hatfield et al, 1982; McPherson et al, 1984; Pitt et al, 1985). However, these studies involved patients undergoing bypass surgeries or palliative small resections. In addition, PTBD-related complications were very common. These flaws in the previous RCTs made it difficult to accept their conclusions. In Western centers, preoperative biliary drainage is performed in selected patients (Laurent et al, 2008; Kennedy et al, 2009). Over time, the bile is contaminated after biliary drainage (Nomura et al, 1999): the incidence of bacterobilia and fungobilia is 85% and 40%, respectively (Jethwa et al, 2007), leading to surgical site infection, cholangitis, and bacteremia after hepatectomy (Hochwald et al, 1999; Jethwa et al, 2007; Nomura et al, 1999; Shigeta et al, 2002). These observations may partly support the limited indications for biliary drainage; however, the mortality rate after extended hepatectomy for jaundiced patients is still high at near 10%, and the cause of death is mainly hepatic failure (Nagino et al, 1993b, 2001). Therefore, preoperative biliary drainage followed by portal vein embolization is recommended before extended hepatectomy, in spite of a lack of clear evidence based on previous RCTs. This is the Japanese standard of care (Henson et al, 2000; Kawasaki et al, 2003).
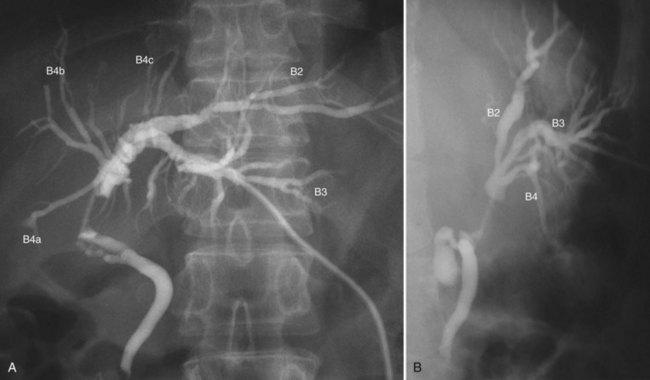
Cholangiography (See Chapter 18)
Cholangiograms precisely delineate the segmental anatomy of the intrahepatic bile duct (Ohkubo et al, 2004), the tumor configuration, and the extent of the tumor border. To avoid misinterpretation related to overlap of segmental ducts, cholangiograms should be taken in various projections (Fig. 50C.5). Most perihilar cholangiocarcinomas are infiltrating tumors, in which cancer cells are likely to extend predominantly in the submucosal layer rather than in the mucosal layer. Histologically, direct and lymphatic invasion are common routes of invasion to the ductal wall, evoking reactive fibrosis (Sakamoto et al, 1998; see Chapter 47). These changes cause rigidity, narrowing, tapering, and obstruction of the duct on the cholangiogram. The border of these findings on cholangiograms correspond to the leading edge of cancer infiltration (Sakamoto et al, 1998).
In hilar biliary obstruction, direct cholangiography frequently shows insufficient opacification of the proximal ductal system. However, MR cholangiography (MRC) delineates the whole biliary system, and its ability to diagnose the Bismuth classification is superior, with an accuracy of 81% (Yeh et al, 2000). Overestimation is a common pitfall of MRC, which may preclude patients from potentially curative surgery (Otto et al, 2004). The limitations of MRC include lower spatial resolution, longer acquisition time, and sensitivity to motion.
Cholangioscopy
Despite the advent of new diagnostic modalities, cholangioscopic examination with biopsy sampling plays an important role in the diagnosis of biliary malignancies (see Chapter 28). Differentiation between malignant and benign lesions, evaluation of proximal and distal cancer extent, and early detection of small lesions and multiple cancer foci can be accomplished by cholangioscopic observation with biopsy sampling (Nimura et al, 1989). Irregularly dilated and tortuous vessels are characteristic of malignancy. A fine granular/papillary mucosa extending continuously from a main lesion is a characteristic finding of superficial spread (Nimura & Kamiya, 1998). Papillary tumors are often associated with the superficial spread of carcinoma (Igami et al, 2009; Sakamoto et al, 1998), and mucosal extension is easily detectable by cholangioscopy. Therefore, cholangioscopy should be considered for patients with papillary tumors. Attention should be paid not only to the intrahepatic extension but also to the distal extension of the tumor.
Percutaneous transhepatic cholangioscopy (PTCS) is performed through the PTBD sinus tract. One week after PTBD, the drainage catheter is replaced by a 9- or 10-Fr PTCS catheter, and then the sinus tract is gradually dilated by replacing the preceding catheter with a larger catheter two or three times a week. A small-caliber cholangioscope (Olympus XP-20) can be used via the sinus tract of the 12-Fr catheter; a standard-type cholangioscope (Olympus, P-20) requires placement of a 15-Fr catheter. This preparation process for PTCS is time consuming and painful; therefore PTCS has recently been replaced by peroral cholangioscopy (POCS). With technical advances in endoscopy, POCS with biopsy gives a high diagnostic accuracy of the extent of the superficial spreading lesion (Kawakami et al, 2009).
Multidetector Row Computed Tomography
Recent advances in helical CT technology, such as multislice detectors and subsecond rotation, have dramatically reduced scanning times in comparison with single-slice CT and have provided unparalleled capability for fast data acquisition and narrow collimation. Thus, multidetector row computed tomography (MDCT) permits the acquisition of three distinct circulatory phases, consisting of the arterial, pancreatic, and portal venous phases in the pancreatobiliary region with 1-mm collimation (Itoh et al, 2003; see Chapter 16). These isotropic volume data allow multiplanar reformation (MPR), which can demonstrate complex anatomic relationships and three-dimensional volume rendering (3DVR) images, such as CT arteriography and CT portography, in a single scanning session (Fig. 50C.6). These postprocessing techniques enhance the diagnostic capability of CT (Choi et al, 2007
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree