Chapter 50B Cancer of the bile ducts
Extrahepatic biliary tumors
Incidence and Etiology
Worldwide, cholangiocarcinoma accounts for approximately 3% of all gastrointestinal cancers. In the United States, where approximately 5000 new cases (gallbladder cancers excluded) are diagnosed each year, the annual incidence of bile duct cancer is estimated at 1 to 2 per 100,000 (Carriaga & Henson, 1995; Greenlee et al, 2001; Khan et al, 2005). Over the past 3 decades, a trend has been seen toward older patient age (median range, 65 to 70 years) at the time of diagnosis and a relative increase in the ratio of intrahepatic to extrahepatic cholangiocarcinoma (see Chapter 50A; Carriaga & Henson, 1995; Endo et al, 2008a; Patel, 2001; Weber et al, 2007).
The molecular changes associated with the pathogenesis of cholangiocarcinoma are poorly characterized (see Chapter 8B). Multiple KRAS oncogene mutations at codon 12 have been identified in almost all resected specimens (Levi et al, 1991). These mutations most likely occur at some point along a stepwise progression to malignancy but probably are not the inciting genetic lesion. There are wide epidemiologic variations in the incidence of KRAS mutations (Lee et al, 1995; Levi et al, 1991; Nakamura et al, 1981). Many studies have reported differences in various genetic abnormalities according to location within the biliary tree, suggesting anatomic differences in pathogenesis. An in-depth review of the molecular biology of biliary cancer is beyond the scope of this chapter; the interested reader is referred to selected review articles (Rashid, 2002) and to Chapter 8B.
The majority of patients with cholangiocarcinoma have no obvious risk factors, although several conditions are associated with an increased incidence of cholangiocarcinoma. In the United States, the most common risk factor for cholangiocarcinoma is primary sclerosing cholangitis (PSC), an autoimmune disease characterized by periductal inflammation resulting in multifocal strictures of the intrahepatic and extrahepatic bile ducts (see Chapter 41; Broome et al, 1996; Katoh et al, 1988; Pitt et al, 1995a, 1995b). Approximately three quarters of patients with PSC have underlying ulcerative colitis (Broome et al, 1996). The natural history of PSC is quite variable, and the true incidence of cholangiocarcinoma is difficult to calculate but appears to depend on disease duration. In a Swedish series of 305 patients followed for more than 5 years, 8% of patients eventually developed cancer (Broome et al, 1996), and occult cholangiocarcinoma has been reported in 40% of autopsy specimens and 36% of liver explants from patients undergoing orthotopic liver transplantation for PSC (Broome et al, 1996; Katoh et al, 1988; Pitt et al, 1995a). Unfortunately, cholangiocarcinoma arising in the setting of PSC frequently is not amenable to operative resection because of advanced underlying hepatic parenchymal disease or multifocal malignancy. Medical or surgical treatment of coexisting ulcerative colitis in patients with PSC does not alter the subsequent risk of developing cholangiocarcinoma (Broome et al, 1996; Katoh et al, 1988; Pitt et al, 1995a).
Choledochal cysts are associated with an increased risk for cholangiocarcinoma (see Chapter 46; Hewitt et al, 1995; Vogt, 1954). The incidence of bile duct cancer in patients with congenital biliary cystic disease is substantial (15% to 20%), especially in patients who are not treated until after age 20 years and in patients previously treated by cyst drainage (Lipsett et al, 1994; Vogt, 1954). The hypothetical risk for malignant transformation of choledochal cysts may be related to an anomalous pancreatobiliary junction, which is commonly found in these patients and predisposes them to reflux of pancreatic juice into the biliary tree and chronic inflammation of the bile ducts (Lipsett et al, 1994; Jeng et al, 1994; Tanaka et al, 1998a; Vogt, 1954). By similar mechanisms, transduodenal sphincteroplasty and endoscopic sphincterotomy may be associated with an increased risk for cholangiocarcinoma (Tanaka et al, 1998b). In a series of 119 patients subjected to sphincteroplasty for benign disease (sphincter of Oddi dysfunction), Hakamada and associates (1997) found a 7.4% incidence of cholangiocarcinoma over 18 years.
Recurrent pyogenic cholangiohepatitis and hepatolithiasis resulting from chronic portal bacteremia and portal phlebitis are prevalent in Japan and parts of Southeast Asia (see Chapters 39 and 44). Although most cholangiocarcinomas in these patients arise within the intrahepatic ducts, cases of extrahepatic cholangiocarcinoma have been reported (Chen et al, 2004; Chu et al, 1997; Kubo et al, 1995; Meade et al, 1994; Winslet et al, 1990). Biliary parasites—specifically, Clonorchis sinensis and Opisthorchis viverrini—are endemic in parts of Asia and are associated with an increased risk of cholangiocarcinoma (Pitt et al, 1995a, 1995b). In Thailand, where approximately 7 million people are chronically infected with Opisthorchis, the annual incidence of cholangiocarcinoma is 87 per 100,000 (see Chapter 45; Watanapa, 1996). In addition, several radionuclides and chemical carcinogens may be associated with an increased risk of cholangiocarcinoma, including thorium, radon, nitrosamines, dioxin, and asbestos.
Tumor Location and Histopathology
Adenocarcinoma is the most common neoplasm arising from the biliary tract and accounts for more than three fourths of all bile duct tumors (Are et al, 2006; Jarnagin et al, 2001). Benign neoplasms and strictures occasionally will mimic many of the clinical, radiographic, and operative findings associated with malignant biliary strictures. Other reported tumors of the extrahepatic bile ducts include adenoma, leiomyoma, paraganglioma, lipoma, granular cell tumor, and carcinoid (Felekouras et al, 2009; Holzinger et al, 1999). Benign strictures resulting from autoimmune pancreatitis, granulomatous disease, and PSC can masquerade as biliary neoplasms and should be treated as such (see Chapter 42A; Corvera et al, 2005; Hadjis & Blumgart, 1985). Malignant strictures of the extrahepatic biliary tree may represent secondary involvement from cancers arising from the gallbladder, pancreas, and periportal lymph nodes (see Chapter 49).
Cholangiocarcinoma can arise anywhere within the biliary tree, but tumors involving the biliary confluence are the most common. Retrospective reviews from high-volume centers reveal that between 40% and 60% of cholangiocarcinoma cases involve the perihilar bile ducts (Burke, 1998; Nagorney et al, 1993; Nakeeb et al, 1996a, 1996b; Tompkins et al, 1981). Tumors of the lower bile duct are classified according to their anatomic location, although there may be considerable overlap. Distal bile duct tumors typically arise within the pancreatic portion of the common bile duct (Tompkins et al, 1981) and represent approximately 20% to 30% of all cholangiocarcinomas and 5% to 10% of all periampullary tumors (Fong et al, 1996; Nakeeb et al, 1996a, 1996b; Wade et al, 1997; Yeo et al, 1997). Tumors of the mid–bile duct are rare and arise below the confluence of the hepatic ducts, usually between the upper border of the pancreas at the level of the cystic duct. The majority of such malignant strictures are caused by gallbladder cancer or cystic duct–based tumors.
Cholangiocarcinomas can present with extensive ductal involvement alone or with early metastatic spread to the liver, regional lymph nodes, or peritoneum (Jarnagin et al, 2005; Tsuzuki et al, 1983). Most bile duct cancers demonstrate characteristic invasive spread along ductal subepithelial histologic planes. Perineural and lymphovascular involvement is common, as is direct invasion into the liver or perihepatic structures (Weinbren & Mutum, 1983).
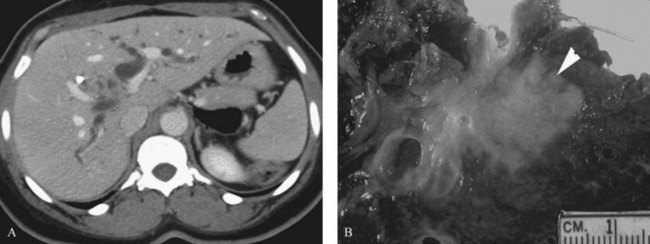
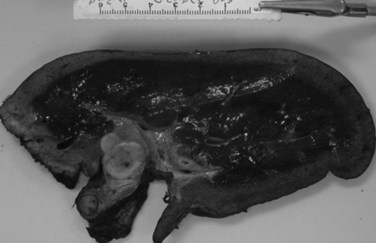
Three distinct macroscopic subtypes of cholangiocarcinoma have been described: 1) sclerosing, 2) nodular, and 3) papillary (Weinbren & Mutum, 1983). Sclerosing tumors account for most cases and are more common at the hilus than in the distal bile duct. They are firm and cause an annular thickening of the bile duct, often with diffuse infiltration and fibrosis of the periductal tissues (Fig. 50B.1). Nodular tumors are characterized by a firm, irregular nodule of tumor that projects into the lumen of the duct. Features of both types are often seen, hence the frequently used descriptor nodular-sclerosing. The papillary tumors account for approximately 10% of all cholangiocarcinomas, and although occasionally seen at the hilus, these are more common in the distal bile duct (Weinbren & Mutum, 1983). These tumors are soft and friable and may be associated with little transmural invasion; a polypoid mass that expands the duct, rather than contracting it (Fig. 50B.2), is a characteristic feature. Although papillary tumors may grow to a significant size and appear surgically unapproachable on radiographic studies, they often arise from a well-defined stalk and can be minimally invasive. The bulk of the tumor frequently is mobile within the bile duct. Recognition of this variant is important, because they appear to be more often resectable and have a more favorable prognosis than the sclerosing type (Jarnagin et al, 2005; Pitt et al, 1995a).
Longitudinal spread along the duct wall and periductal tissues is an important pathologic feature of cholangiocarcinomas (Endo, 2008b; Sakamoto et al, 1998; Weinbren & Mutum, 1983). There may be substantial extension of tumor beneath an intact epithelium, and it can extend as much as 2 cm proximally and 1 cm distally (Sakamoto et al, 1998; Shimada et al, 1988). The full tumor extent may be underestimated by radiographic studies and may not be appreciated on palpation. This predilection for submucosal extension underscores the importance of frozen-section analysis of the duct margins during resection, although its utility has been called into question (Endo et al, 2008b). Although most of these tumors are adenocarcinomas, in rare instances other malignant cell types may arise primarily in the biliary tree and cause biliary obstruction, such as carcinoid tumors (Jutte et al, 1987; Rugge et al, 1992; Sankary et al, 1995).
Clinical Presentation
The early symptoms of hilar and distal cholangiocarcinoma are nonspecific. Abdominal pain, anorexia, weight loss, and pruritus are the most common symptoms, but these are seen only in about one third of patients. Fever is rarely seen at initial presentation but is common once biliary manipulation is initiated (Farley et al, 1995; Katoh et al, 1988; Meade et al, 1994; Nakeeb et al, 1996b; Pitt et al, 1995a; Vatanasapt et al, 1990b). Most patients have few symptoms and come to attention because of jaundice or abnormal liver function tests. Although most patients eventually become jaundiced, this finding is not present initially in cases of incomplete biliary obstruction (i.e., right or left hepatic duct) or segmental ductal obstruction. Segmental obstruction may go unrecognized for a long time, resulting in ipsilateral lobar atrophy without overt jaundice (see Chapter 5). These patients often are further evaluated and diagnosed because of an elevated serum alkaline phosphatase.
The clinical presentation of distal bile duct cancer generally is indistinguishable from that of other periampullary malignancies. Progressive jaundice is seen in 75% to 90% of patients. Abdominal pain, weight loss, fever, or pruritus occur in one third of patients or fewer (Fong et al, 1996; Nakeeb et al, 1996a, 1996b).
In jaundiced patients, the total bilirubin level may suggest an etiology. In patients with obstruction from cholangiocarcinoma, the serum bilirubin level is often greater than 10 mg/dL and averages 18 mg/dL, whereas patients with obstruction from choledocholithiasis have bilirubin levels 2 to 4 mg/dL and rarely greater than 15 mg/dL (Way, 1994); however, there is likely to be considerable overlap. Furthermore, the degree of jaundice may suggest the level of bile duct obstruction. Hilar obstruction is typically associated with higher levels of serum bilirubin compared with distal tumors. In patients with cholangiocarcinoma and no previous biliary intervention, cholangitis is uncommon at initial presentation despite a 30% incidence of bactibilia (see Chapter 43; Hochwald et al, 1999; Meade et al, 1994). The incidence of bactibilia is nearly 100% after biliary intubation, and cholangitis is also more common (Hochwald et al, 1999). Gallstones or common bile duct stones may coexist with bile duct cancer. In the absence of certain predisposing conditions (PSC, choledochal cyst, hepatolithiasis), however, it is uncommon for choledocholithiasis to cause obstruction at the biliary confluence. It is imperative to investigate fully and delineate the level and nature of any obstructing lesion causing jaundice to avoid missing the diagnosis of carcinoma.
Bactibilia is relatively common in patients with hilar cholangiocarcinoma; however, in the absence of prior biliary manipulation, frank cholangitis is uncommon at initial presentation (McPherson et al, 1984).
Diagnostic Studies
The diagnosis of hilar and distal cholangiocarcinoma usually follows targeted studies for the evaluation of obstructive jaundice or elevated liver enzymes. Most patients are referred after having had some studies done elsewhere, usually computed tomography (CT) and often some form of direct cholangiography, such as percutaneous transhepatic cholangiography (PTC) or endoscopic retrograde cholangiopancreatography (ERCP). In our view, histologic confirmation of malignancy is not mandatory before exploration. In the absence of previous biliary tract surgery, the finding of a focal stenotic lesion combined with the appropriate clinical presentation is sufficient for a presumptive diagnosis of hilar cholangiocarcinoma, which is correct in most instances (Wetter et al, 1991).
Although most patients with hilar strictures and jaundice have cholangiocarcinoma, alternative diagnoses are possible in 10% to 15% of patients (Wetter et al, 1991). The most common of these are gallbladder carcinoma, Mirizzi syndrome, and benign focal strictures, such as lymphoplasmacytic sclerosing pancreatitis/cholangitis, granulomatous disease, and PSC (see Chapter 42A, Chapter 42B, Chapter 48, Chapter 49 ). Distinguishing gallbladder carcinoma from hilar cholangiocarcinoma can be difficult. A thickened, irregular gallbladder wall with infiltration into segments IV and V of the liver, selective involvement of the right portal pedicle, and obstruction of the mid–bile duct with occlusion of the cystic duct on endoscopic cholangiography all suggest gallbladder carcinoma (Fig. 50B.3).
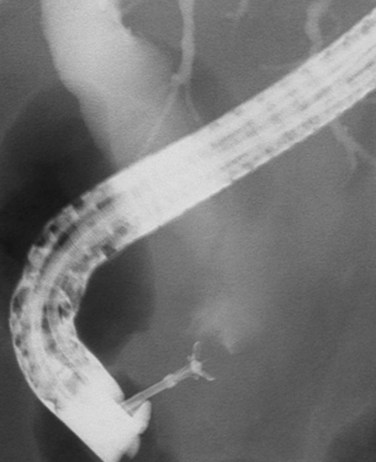
Mirizzi syndrome is a benign condition resulting from a large gallstone impacted in the neck of the gallbladder (see Chapters 29 and 42A). The ensuing pericholecystic and periductal inflammation and fibrosis can obstruct the proximal bile duct, which is often difficult to distinguish from a malignant obstruction (Baer et al, 1990; Callery et al, 1997; Cabooter et al, 1990). Other benign focal strictures (malignant masquerade) can occur at the hepatic duct confluence but are uncommon (Fig. 50B.4; Hadjis et al, 1985; Saldinger & Blumgart, 2000; Verbeek et al, 1992; Wetter et al, 1991). The finding of a smooth, tapered stricture on cholangiography suggests a benign stricture. Diagnostic assessments based on the cholangiographic appearance of the stricture are unreliable, and hilar cholangiocarcinoma must remain the leading diagnosis until definitively disproved with operative exploration. The alternative conditions that one may encounter are best assessed and treated at operation. Relying on the results of percutaneous needle biopsy or biliary brush cytology is dangerous, because they often are misleading, and the surgeon may miss the opportunity to resect an early cancer (Enjoji et al, 1998; Rabinovitz et al, 1990). In the absence of clear contraindications, exploration is indicated in all patients with suspicious hilar lesions with concordance of clinical and radiographic findings.
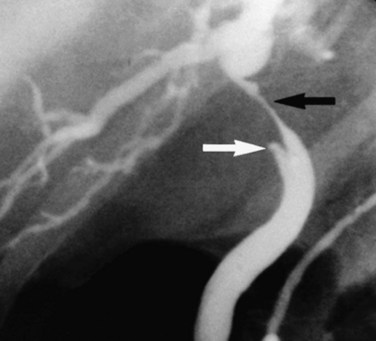
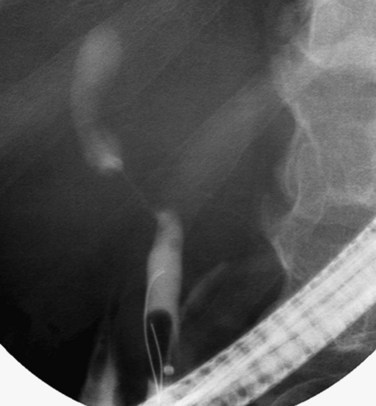
FIGURE 50B.4 Endoscopic retrograde cholangiopancreatography from a patient with a benign stricture of the proximal bile duct. The smooth, tapered appearance (black arrow) is in contrast to the irregular stricture typical of a sclerosing tumor. Nonfilling of the cystic duct (white arrow) is an important finding, and in the appropriate setting, it should raise the suspicion of gallbladder carcinoma (see Fig. 50B.3). In this patient, the chronic inflammatory process had involved the hepatic duct and cystic ducts. Most cases of benign strictures of the proximal bile duct spare the cystic duct.
Many tumors express carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9). Serum levels of these markers, although elevated in some patients, have had little diagnostic value (Pitt et al, 1995a). It has been suggested that CEA levels in hepatic bile may help distinguish between benign and malignant strictures in patients with premalignant conditions (Nakeeb et al, 1996a).
Direct cholangiography (see Chapter 18) shows the location of the tumor and the biliary extent of disease, both of which are crucial in surgical planning. When performed skillfully, ERCP provides useful duct detail and can effectively drain functional liver segments prior to operative planning. Although invasive, PTC outlines the extent of intrahepatic bile duct involvement reliably and has been the preferred study in the past (Pitt et al, 1995a, 1995b). High-quality cholangiograms are often thought to be essential for accurately diagnosing the extent of cancer (Kondo et al, 2004; Nimura, 1997). In many centers, particularly in Japan, surgeons extend the diagnostic process by performing detailed cholangiography with one or more percutaneous catheters placed in the intrahepatic ducts, often combined with percutaneous cholangioscopy along a mature transhepatic drain tract (Ohkubo et al, 2004; Ozden, 2002). PTC with biliary drainage should be considered not only an effective method of relieving jaundice but also an important diagnostic modality.
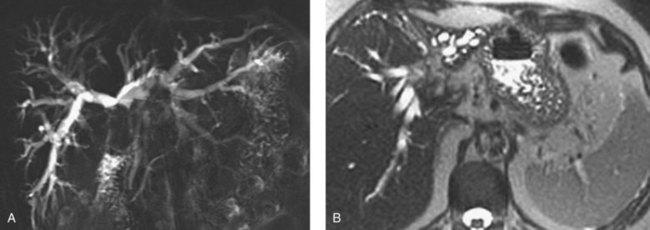
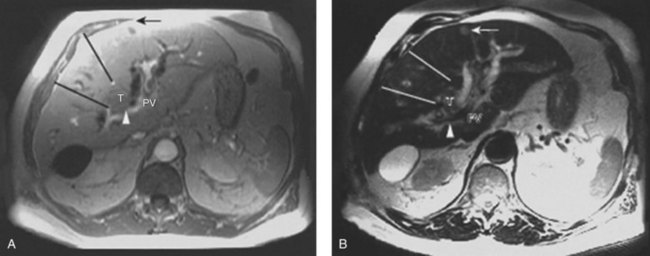
MRCP is a powerful investigative tool that has almost replaced endoscopic and percutaneous cholangiography as the initial preoperative assessment of hilar cholangiocarcinoma (see Chapter 17). Several studies have shown its utility in evaluating patients with biliary obstruction (Guthrie et al, 1996; Lee et al, 1997; Schwartz et al, 1998). MRCP may not only identify the tumor and the level of biliary obstruction, but it also may reveal obstructed and isolated ducts not appreciated at endoscopic or percutaneous study (Fig. 50B.5). MRCP also provides information regarding the patency of hilar vascular structures, the presence of nodal or distant metastases, and the presence of lobar atrophy (Fig. 50B.6).
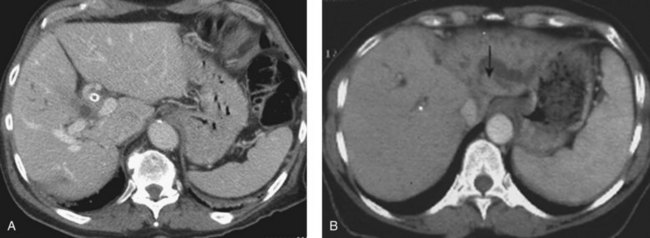
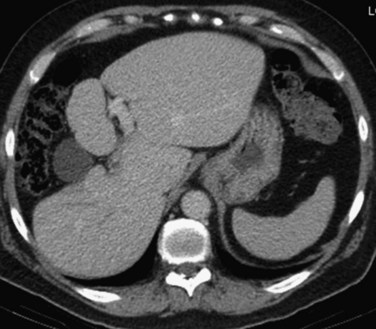
CT is an important study for evaluating patients with biliary obstruction. A high-quality CT scan can provide valuable information regarding the level of obstruction, vascular involvement, and liver atrophy (Fig. 50B.7; see Chapter 16). Advances in CT technology permit the acquisition of three distinct circulatory phases—the arterial, pancreatic, and portal venous phases—in the hilar and pancreatobiliary region with 1-mm collimation. These isotropic volume data allow generation of high-quality three-dimensional images—such as CT arteriography, CT cholangiopancreatography, and CT portography—in a single scanning session (Itoh et al, 2005).
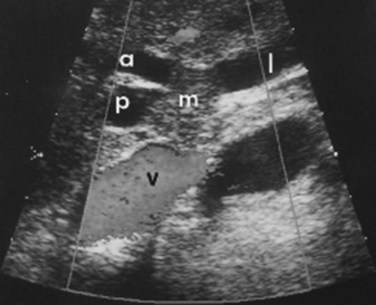
Although it is operator dependent, ultrasound (US) is a useful noninvasive diagnostic study (see Chapter 13). When utilized by experienced centers, it often delineates tumor extent accurately. US can not only show the level of biliary ductal obstruction, but it can also provide information regarding tumor extension within the bile duct and in the periductal tissues (Gibson et al, 1986; Hann et al, 1997; Okuda et al, 1988). Duplex US is firmly established as a highly accurate predictor of vascular involvement and resectability (Fig. 50B.8). In a series of 19 consecutive patients with malignant hilar obstruction, US with color spectral Doppler technique was equivalent to angiography and CT portography in diagnosing lobar atrophy, level of biliary obstruction, hepatic parenchymal involvement, and venous invasion (Hann et al, 1997). Duplex US is particularly useful for assessing portal venous invasion. In a series of 63 consecutive patients from Memorial Sloan-Kettering Cancer Center (MSKCC), duplex US predicted portal vein involvement in 93% of cases, with a specificity of 99% and a positive predictive value of 97%. In the same series, angiography with CT angioportography had a 90% sensitivity, 99% specificity, and 95% positive predictive value (Bach et al, 1996).
The diagnosis of malignancy in patients with bile duct strictures can be challenging and depends on accurate interpretation of ERCP and/or PTC as well as cross-sectional imaging (MRCP and CT) findings. ERCP in particular is a useful tool for assessing biliary tract strictures, as it permits visualization of the biliary tree with the opportunity for therapeutic intervention (biliary drainage) and collection of specimens for cytopathologic and histopathologic evaluation (Fig. 50B.9). Although highly specific, biliary brush cytology is limited by low to moderate sensitivity (15% to 68%; Fritcher et al, 2009). As a result, adjunctive diagnostic techniques including mutation analysis, DNA ploidy analysis, and fluorescence in situ hybridization (FISH) have been studied clinically and experimentally to improve tumor detection sensitivity (Kipp et al, 2010). Two types of chromosomal abnormalities are frequently identified with a clinical FISH probe set, namely polysomy and trisomy 7. Polysomy has been defined as a gain of two or more of the four probes in five cells. A large clinical study recently demonstrated that FISH was more sensitive than cytology for detecting biliary tract cancer and was able to detect 49 (22%) of 227 cancers that routine cytology interpreted as nonmalignant, without compromising test specificity (Fritcher et al, 2009).
Distal bile duct tumors frequently are mistaken for adenocarcinoma of the pancreatic head, the most common periampullary malignancy. In a series of 119 periampullary tumors, the site of origin was diagnosed incorrectly in 28% of patients preoperatively and in 20% of patients intraoperatively (Jones et al, 1985). Nevertheless, ERCP can provide valuable information regarding the level of obstruction and may show clearly that the obstruction is arising from the bile duct and does not involve the pancreatic duct. ERCP also may be useful in cases in which choledocholithiasis is suspected and may be therapeutic in these patients. PTC is generally less useful for tumors of the distal bile duct. A good quality cross-sectional imaging study also is required, usually CT, to assess for vascular involvement and metastatic disease. CT commonly does not reveal a mass, however, because these tumors are frequently small at the time of presentation. MRCP increasingly is being used to evaluate periampullary tumors. As is true for hilar lesions, it can provide images of the distal bile duct previously obtainable only with direct cholangiography and without the need for invasive procedures (Georgopoulos et al, 1999). A dilated extrahepatic bile duct terminating abruptly at its distal aspect without a concomitantly dilated pancreatic duct suggests a distal bile duct carcinoma.
In patients with a stricture of the distal bile duct and a clinical presentation consistent with cholangiocarcinoma, histologic confirmation of malignancy is generally unnecessary, unless nonoperative therapy is planned. Benign strictures occur in the lower bile duct, but these are difficult to differentiate definitively from malignant strictures without resection. Percutaneous needle biopsy is difficult, often impossible, because of the small size of these tumors. In addition, endoscopic brushings of the bile duct have an unacceptably low sensitivity, making a negative result virtually useless (Ryan, 1991). Reliance on the results of percutaneous endoscopic US-guided fine needle aspiration or endoscopic brush biopsy serves only to delay operative therapy.
Preoperative Evaluation and Management
When a biliary tract malignancy is suspected on clinical and radiographic grounds, patient evaluation should turn toward an assessment of tumor resectability and of hepatic and overall physiologic reserve. The presence of significant comorbid conditions, chronic liver disease, or portal hypertension generally precludes resection. In patients not fit for operation, biliary drainage is an appropriate intervention to consider, and the diagnosis should be confirmed histologically if palliative therapies focused on local and systemic disease control are anticipated. Patients with potentially resectable tumors occasionally present with biliary tract sepsis, frequently after instrumentation of the biliary tree. These patients require resuscitation and treatment of the infection before operation can be considered. The preoperative evaluation must address the four crucial determinants of resectability: 1) extent of tumor within the biliary tree, 2) vascular involvement, 3) hepatic lobar atrophy, and 4) metastatic disease (Burke, 1998; Jarnagin et al, 2005).
Lobar atrophy is an often overlooked or underappreciated finding in patients with hilar cholangiocarcinoma; however, its importance in determining resectability cannot be overemphasized, because it often influences therapy (Hadjis & Blumgart, 1987; see Chapter 5). Long-standing biliary obstruction may cause moderate atrophy, whereas concomitant portal venous compromise induces rapid and severe atrophy of the involved segments. On cross-sectional imaging, atrophy is characterized by a small, often hypoperfused lobe with crowding of the dilated intrahepatic ducts (Figs. 50B.10 and 50B.5B). The crucial determinants of resectability, including lobar atrophy, can be assessed with noninvasive studies that include cross-sectional imaging. Tumor involvement of the portal vein is usually present if compression, narrowing, encasement, or occlusion is seen on imaging studies.
Preoperative Biliary Drainage
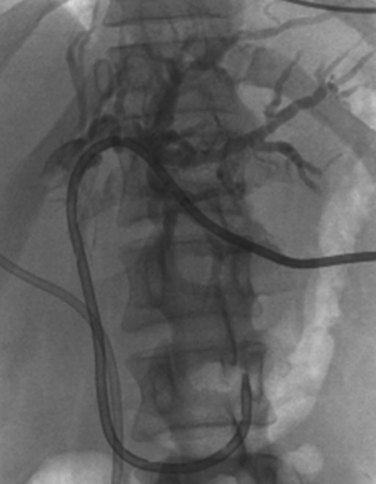
Typically, patients with hilar cholangiocarcinoma will require major liver resection, so their preoperative management must be optimized carefully. Obstructive jaundice associated with perihilar cholangiocarcinoma differs from that associated with middle or distal bile duct cancer. In distal bile duct cancer, a single catheter is enough for biliary drainage, whereas in perihilar cholangiocarcinoma, multiple biliary drainage catheters of the future liver remnant may be necessary. Experienced centers have demonstrated excellent results with endoscopic biliary drainage, but controversy exists as to whether this technique should replace percutaneous transhepatic biliary drainage (Gholson & Burton, 1991; Polydorou et al, 1992; Speer et al, 1987). Endoscopic retrograde biliary drainage offers several advantages, including low procedure-related morbidity and mortality, shorter hospital stay, and improved patient comfort. The limitations of endoscopic retrograde biliary drainage include a high incidence of cholangitis, especially when multiple obstructed ducts are instrumented but not subsequently drained (Andersen et al, 1989; Chang et al, 1998; Liu et al, 1998; Rerknimitr et al, 2004), and the difficulty in follow-up assessment of the extent of segmental duct involvement once an endobiliary stent is placed. Furthermore, control of which exact segmental ducts are cannulated is imperfect when performed endoscopically. For both endoscopic and percutaneous biliary drainage, a few guiding principles should be followed. The intrahepatic bile duct of the predicted future liver remnant (FLR) is intubated or punctured (Kawasaki et al, 2003; Seyama et al, 2003) and subsequently drained. If the FLR cannot be predicted with certainty in the preoperative setting, the left hepatic duct should be manipulated and drained (Fig. 50B.11). When the intrahepatic bile ducts are isolated into segmental units, PTC and multiple direct segmental transhepatic drainages may be necessary (Nagino et al, 1992; Nimura et al, 1995). If segmental cholangitis develops in patients with endoscopic or transhepatic biliary drainage catheters, urgent drainage of affected ducts should be attempted (Iyomasa et al, 1994; Sakamoto et al, 1995).
Clinical studies of biliary decompression have shown that external drainage has limited benefit, and that by preserving intestinal bile flow, the internal drainage is associated with more advantages (Clements et al, 1993). Superiority of internal drainage was shown in experimental studies, which showed that rat liver regeneration after partial hepatectomy is suppressed under external drainage but not under internal drainage (Saiki et al, 1999; Suzuki et al, 1994). Ogata and colleagues (2003) investigated bacterial translocation in bile duct–ligated rats, finding that bile replacement is essential to maintain intestinal barrier function. Impaired intestinal barrier function does not recover by percutaneous biliary drainage (PBD) without bile replacement. Bile replacement can restore the intestinal barrier function primarily as a result of repair of physical damage to the intestinal mucosa. Bile replacement during external biliary drainage should be recommended for planned hepatectomy for hilar cholangiocarcinoma because of the substantial infectious morbidity associated with these resections.
Many retrospective reports (Denning et al, 1981; Gundy et al, 1994; Nakayama et al, 1978; Norlander et al, 1982) support preoperative biliary drainage, whereas several older, prospective randomized studies (Hatfield et al, 1982; McPherson et al, 1984; Pitt et al, 1985) concluded that drainage did not reduce overall morbidity and mortality following management of proximal cholangiocarcinoma. The pertinence of the these cited randomized studies is questionable, as few major hepatectomies were included in these trials, and preoperative biliary drainage procedures were associated with unexpectedly high morbidity and mortality rates. In addition to accurate assessment of bile duct involvement, preoperative biliary drainage improves cholestatic liver disease and affords safer performance of hepatectomy for hilar cholangiocarcinoma. Lastly, the volume of the future liver remnant should be assessed prior to drainage, because with an atrophic hemiliver, very little functional liver may be removed with the resection, and performance of the operation without drainage should be considered (Kennedy et al, 2009).
Three-Dimensional Computed Tomography
Advances in helical CT technology, such as multiple simultaneous detectors and subsecond rotation, have shown unparalleled capabilities for fast data acquisition and narrow collimation (see Chapter 16). Multidetector CT permits the acquisition of three distinct circulatory phases—arterial, portal, and late venous—in the pancreatobiliary and intrahepatic regions with 1-mm collimation. These isotropic volume data allow generation of high-quality three-dimensional images with CT arteriography, CT cholangiopancreatography, and CT portography in a single scanning session (Itoh et al, 2005).
CT is now a mainstay in the preoperative diagnostic evaluation for patients with perihilar cholangiocarcinoma, and it provides information on resectability and planning of the operation. Three-dimensional understanding of the main hepatic artery distribution may facilitate skeletonization of the hepatoduodenal ligament, and understanding of the intrahepatic artery branching is helpful during resection of the segmental or subsegmental bile ducts as the final step of resection. Hepatobiliary surgeons must appreciate two variations of the hepatic artery distribution: One is the left hepatic artery running right to the umbilical portion of the left portal vein, the other is a posterior branch of the right hepatic artery running cranially to the right portal trunk. Careful attention should be paid to the former variant in extended right hepatectomy with caudate lobectomy, and the same applies to the latter variant in extended left hepatectomy with caudate lobectomy (see Chapter 1B).
Unilateral involvement of the hepatic artery is compatible with resection (Williamson et al, 1980). Bilateral involvement of the hepatic artery usually is associated with nonresectability, when the tumor is located mainly on the right. Hepatic arterial reconstruction to accomplish a complete resection should be reserved for highly selected patients with potentially curable disease. Similarly, although not an absolute contraindication for resection of hilar cholangiocarcinoma, main portal vein invasion will preclude a complete resection with long-term benefit for the majority of patients (Blumgart et al, 1984; Hoevels & Ihse, 1979; Launois et al, 1979; Williamson et al, 1980). Despite the poor prognosis associated with main portal vein invasion in general, several groups have reported reasonable long-term outcomes with portal vein resection for advanced biliary cancer (Ebata et al, 2003; Miyazaki et al, 1998a; Nimura et al, 1991a; Sakaguchi & Nakamura, 1986).
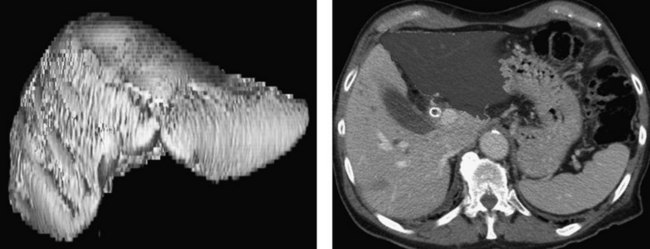
Three-dimensional CT also offers the capacity to generate volumetric models of the total liver for planned hepatic resection and FLR (Fig. 50B.12). Because patients subjected to resection of more than 75% of functional liver parenchyma have substantially higher risk of postoperative liver failure regardless of existing cholestatic liver disease, routine preoperative volumetry may help to identify and select candidates for biliary drainage and portal vein embolization (PVE) who may be at considerable risk for postoperative morbidity and mortality.