CHAPTER 8
Biological agents
Biological agents approved to treat IBD
All of the biological agents currently approved for the treatment of inflammatory bowel disease (IBD) are monoclonal antibodies (Table 8.1). Two antitumor necrosis factor (TNF) IgG1 antibodies, infliximab (Remicade®) and adalimumab (Hunira®), and one pegylated Fab antibody fragment, certolizumab-pegol (Cimzia®), have demonstrated efficacy in the treatment of refractory luminal Crohn’s disease (CD). Infliximab is a chimeric mouse-human antibody, certolizumab is a humanized Fab fragment. Golimumab (Simponi®) and adalimumab are fully human antibodies. The human TNF receptor-Fc fragment construct, etanercept (Enbrel®), has failed to show efficacy in Crohn’s disease. The pleotropic inflammatory cytokine, TNF, is secreted by several immune cells, predominantly by monocytes and, after binding to one of its 2 receptors, induces several pro-inflammatory signals in immune and nonimmune cells. Inhibition of TNF signalling results in apoptosis of activated T-cells, decreased cytokine secretion, reduction of leukocyte migration and restoration of the mucosal barrier of the gut. Only infliximab is approved by the European and American authorities to treat perianal fistulizing CD. Also, scheduled maintenance therapy with infliximab and adalimumab results in more pronounced mucosal healing and is associated with a reduction in disease-related hospitalizations. In ulcerative colitis (UC), infliximab, adalimumab and golimumab induce and maintain remission and result in mucosal healing in treatment-refractory moderate-to-severe disease. In addition, infliximab is efficacious in decreasing the risk of colectomy in steroid refractory hospitalized patients with severe attacks of UC.
Table 8.1 Biological agents approved for IBD with their respective recommended dosing
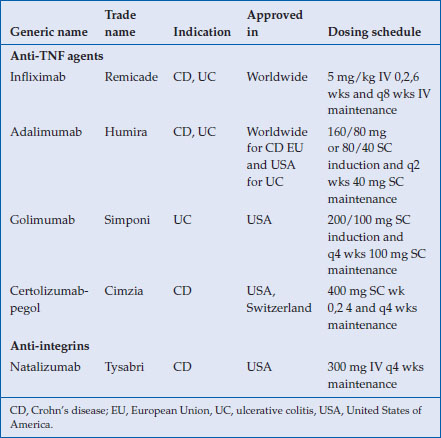
Infliximab is administered intravenously at a dose of 5 mg/kg at week 0, 2 and 6 for induction and afterwards every 8 weeks as maintenance therapy. Adalimumab induction therapy consists of two doses given subcutaneously (SC) 2 weeks apart. Depending on disease severity, 80/40 mg or 160/80 mg is used at induction. Maintenance therapy consists of 40 mg administered every 2 weeks. Golimumab has been approved by the FDA in May 2013 for the induction and maintenance of ulcerative colitis at a dose of 200 mg SC, followed by 100 mg SC after 2 weeks and 100 mg maintenance every 4 weeks thereafter. Approval in other jurisdictions around the world is pending.
For children and adolescents with IBD, anti-TNF antibodies have become available more recently. Currently, infliximab and adalimumab are approved in the USA, Canada and in Europe for pediatric CD and infliximab for pediatric UC. Dosing per kilogram is identical to that used in adults. Since adalimumab dosing is not weight-based, the induction and maintenance doses are adapted for children who are underweight. Certolizumab-pegol has not been studied for pediatric IBD.
Natalizumab (Tysabri®), an anti-α4 integrin IgG4 antibody and the first representative of a new class of biological agents, the anti-adhesion molecules, maintains steroid free clinical remission in luminal Crohn’s disease. The drug is efficacious in patients failing anti-TNF therapy. However, due to the risk of progressive multifocal encephalopathy (PML), a potentially lethal brain infection caused by JC virus reactivation, the FDA has approved its use only for patients failing anti-TNF therapy and in a tight pharmacovigilance program. The antibody has not been approved by Health Canada or by the European Medicines Agency (EMA). Several, more gut-selective, anti-adhesion molecules are in clinical development and clinical efficacy has been shown in ulcerative colitis for the IgG2 monoclonal antibody, vedolizumab.
Optimal treatment strategies with anti TNF therapies in IBD
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







