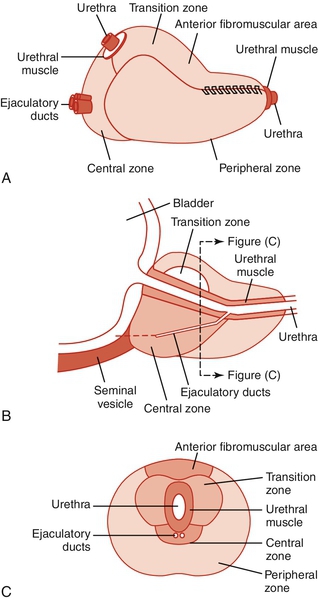
Chapter 15 Stephen Mock, MD; Roger Dmochowski, MD, MMHC, FACS The evaluation and management of symptoms related to bladder outlet and urethral obstruction are responsible for a large portion of any given urology practice. An etiologic categorization is seen in Table 15-1. Although some of these entities may be associated with abnormalities of the urinary sediment or a characteristic finding on physical examination, most present only with lower urinary tract symptoms (LUTS). The symptoms are remarkably nonspecific and are associated more so with some entities rather than others strictly because of their prevalence. This chapter considers the most common of these, benign prostatic hyperplasia (BPH), and its related entities: benign prostatic enlargement (BPE) and benign prostatic obstruction (BPO). Bladder neck and smooth sphincter dyssynergia or dysfunction and striated sphincter dyssynergia have been previously considered in Chapter 14, and urethral stricture disease in Chapter 11. BPH refers to a regional nodular growth of varying combinations of glandular and stromal proliferation that occurs in almost all men who have testes and who live long enough. Because of the anatomic localization of the prostatic growth that characterizes BPH — surrounding and adjacent to the proximal urethra — clinical problems can result. BPH can be defined in a number of ways, depending on the orientation of the user of the term. Microscopic BPH refers to the histologic evidence of cellular proliferation. Macroscopic BPH refers to organ enlargement due to the cellular changes. BPH histopathologically is characterized by an increased number of epithelial and stromal cells in the periurethral area of the prostate, the molecular etiology of which is uncertain. The incidence of histologic or microscopic BPH is far greater than that of clinical or macroscopic BPH. BPH has also been referred to as hyperplasia, benign prostatic hypertrophy, adenomatous hypertrophy, glandular hyperplasia, and stromal hyperplasia. Historically, the term prostatism was applied to almost all symptoms that reflected a micturition disorder in the older man. The term unfortunately implied that the cause of the problem was the prostate, which, in later years was found clearly not to be the case in many instances. The World Health Organization (WHO) sponsored consultations on BPH and has recommended changes to the terminology related to urinary symptoms and the prostate in elderly men. The term LUTS (lower urinary tract symptoms) was introduced by Paul Abrams and has been adopted as the proper terminology to apply to any patient, regardless of age or sex, with urinary symptoms but without implying the underlying problem. LUTS were initially divided into “irritative” and “obstructive” symptoms, but it became obvious that there was a poor correlation between so-called obstructive symptoms and a urodynamic diagnosis of bladder outlet obstruction (BOO) and also between so-called irritative symptoms and a urodynamic diagnosis that related to definable abnormalities seen during filling and storage. Additionally, the term irritative implied to some people an infectious or inflammatory process. Thus the division of LUTS into “filling/storage symptoms and emptying/voiding symptoms” evolved. (See Chapter 14.) The terminology with respect to prostate characteristics has also changed. Paul Abrams was the first to suggest a reconsideration of the use of the term BPH and a redefinition of the terminology. He pointed out that BPH was a histologic diagnosis that had been shown to occur in 88% of men older than 80 years. He added that, in some patients, the prostate gland enlarged, and this condition should be distinguished from BPH and referred to as BPE. In approximately half of these patients with BPE, he stated that true BOO resulted, a condition that should be termed BPO. BPH was previously an all-encompassing term that included prostate size, benign prostate histology, and all filling/storage or voiding/emptying symptoms thought to be related to the prostate pathophysiologically in the adult male. The current terminology recognizes the imprecise and misleading implications of the initial usage of this phrase. The terminology related to the prostate is currently expressed as follows: 2. BPE — benign prostatic enlargement: This refers to the size of the prostate, specifically the prostatic enlargement due to a benign cause, generally histologic BPH. If there is no prostatic histologic examination available, the term prostatic enlargement should be used. 3. BPO — benign prostatic obstruction: This is a form of BOO. This term may be applied when the cause of the outlet obstruction is known to be BPE due to a benign cause, generally histologic BPH. BOO is a functional term for any cause of subvesical obstruction. The WHO-sponsored consultation on BPH recommended the generic phrase LUTS suggestive of BPO to describe elderly men with filling/storage or voiding/emptying problems likely to be caused by an obstructing prostate. It should be noted that there are other causes of BOO than prostatic enlargement (see Table 15-1). Autopsy data indicate that anatomic (microscopic) evidence of BPH is seen in about 25% of men 40 to 50 years of age, 50% of men ages 50 to 60, 65% of men ages 60 to 70, 80% of men ages 70 to 80, and 90% of men ages 80 to 90. Estimates of the prevalence of clinical BPH vary widely, probably because of the varying thresholds used to define the presence of BPH on the basis of symptoms and/or urodynamics (no uniform definition) or on the basis of the rate of prostatic surgery. It has been classically stated that 25% to 50% of individuals with microscopic and macroscopic evidence of BPH will progress to clinical BPH. Depending on which definition is used, the prevalence of clinical BPH in an individual community in men ages 55 to 74 years may vary from less than 5% to more than 30%. Only 40% of this group, however, complain of LUTS, and only about 20% seek medical advice because of them. The number of individuals who receive treatment for clinical BPH varies according to the threshold for providing such treatment, a threshold that can vary widely in different parts of the world and in different parts of the United States. As treatments become less invasive, this number can be expected to rise. Only age and the presence of testes are positively correlated with the development of BPH. Obesity may be positively correlated with prostate volume. Most agree that cirrhosis is inversely correlated, probably because of decreased plasma testosterone (T) levels. A positive association between LUTS secondary to BPO and erectile dysfunction (ED) seems to be real, but whether this is simply age related or not is less certain. There are no consistent correlations for dietary factors, vasectomy, prior sexual history, smoking, or other disease states. BPH does appear to have an inheritable genetic component, although the specifics are yet to be elucidated. Although some prostatic growth occurs throughout life, the prostate changes relatively little in size until puberty, when it undergoes rapid growth. Autopsy studies indicate that the normal adult prostate plateaus at a volume of approximately 25 mL at age 30. This remains relatively stable until approximately age 50, after which increasing volume is observed, such that the average prostate volume is approximately 35 to 45 mL at age 80. Throughout developmental life the prostate maintains its ability to respond to endocrine signals, undergoes rapid growth at puberty, and maintains its size and tissue androgen-receptor levels. In some individuals, abnormal growth subsequently occurs, which may be either benign or malignant. The mechanisms of normal and abnormal growth have yet to be resolved but are thought to involve multiple growth promoting and inhibiting factors ultimately controlling cell replication, cell cycle control, cell aging, cell senescence, and cell death, both necrosis and apoptosis. A full discussion of the factors relating to prostate physiology and growth can be found in Campbell-Walsh Urology, 10th edition, Chapter 90, “Development, Molecular Biology, and Physiology of the Prostate.” The size of the prostate is not linearly correlated to either urodynamic evidence of BOO or the severity of symptoms. The adult prostate is a truncated cone with its base at the urethrovesical junction and its apex at the urogenital diaphragm. The prostate is pierced by the urethra, which angles forward at the verumontanum, and by the paired ejaculatory ducts, which join the urethra at its point of angulation. A lobular configuration of the prostate was originally described by Lowsley, based on studies of the human fetal prostate. A posterior, two lateral, an anterior, and a middle lobe were described. Although this description was used by urologists for years because it seemed to bear some relationship to endoscopic and gross surgical anatomy, distinct lobes do not exist in the prepubertal and normal adult prostate. The concept of a lobular structure has been replaced by one based on concentric zones that have morphologic, functional, and pathologic significance. McNeal and associates from Stanford have done the most to expand our understanding of adult prostate morphology, describing the zonal anatomy based on examination of the gland in different planes of section (Figure 15-1). The urethra represents the primary reference point, dividing the prostate into an anterior fibromuscular and a posterior glandular portion. The anterior fibromuscular stroma comprises up to one third of the total bulk of the prostate. It contains no glandular element. This fibromuscular stroma has not been linked to a specific pathologic process. The two principal regions of the glandular prostate are defined as the peripheral zone (approximately 75% of total glandular volume) and the central zone (approximately 25%), each morphometrically distinct. The central zone makes up about 25% of the functioning glandular prostate. It contains the urethra only at the upper end of the veru, where its ducts open. The development of carcinoma is relatively uncommon in this area. The peripheral zone is the site of origin of most prostate cancer. The glandular tissue that participates in the BPH nodule formation is derived exclusively from the branches of a few small ducts, representing approximately 5% to 10% of the glandular prostate, that join the urethra at or proximal to its point of angulation. Urethral angulation at the most proximal extent of the verumontanum displaces the proximal urethral segment from the secretory gland mass anteriorly and into the anterior fibromuscular stroma. The resulting space between the urethra and glandular prostate accommodates a cylindrical smooth muscle sphincter that surrounds the proximal segment of the urethra from the base of the verumontanum to the bladder neck. All nodules of BPH develop within or immediately adjacent to this smooth muscle layer, and this tissue is subdivided by this muscle into two discrete regions. The transitional zone comprises less than 5% to 10% of the total glandular volume and consists of two separate lobules of tissue immediately outside of the smooth muscle layer, located laterally and extending somewhat ventrally. A tiny periurethral region (less than 1% of the total glandular prostate) contains glands that are entirely contained within the smooth muscle layer from just proximal to the point of urethral angulation to the bladder neck. This periurethral zone is so small that it is not pictured in many other renditions of McNeal’s zonal anatomy. The origin of BPH is confined exclusively to these areas, and some cancers may also originate here. Between the transitional and peripheral zones are the central zones, which have not been implicated in the origin of a specific pathologic process. Clinically detectable BPH nodules arise from a variety of adenomas in the transitional and periurethral zones. As these grow, they may outwardly compress the anterior fibromuscular stroma and areas in the peripheral and central zones. A so-called surgical capsule develops between the hyperplastic nodules and the compressed glandular tissue and serves as a plane of cleavage. This serves as a useful landmark in open or transurethral surgical treatment. The etiologic factors responsible for BPH nodule induction and further development are unclear. However, a number of factors are obviously involved, although the magnitude of their importance and their interactions remains to be fully elucidated. What follows is the briefest of descriptions of the major factors mentioned, gleaned mostly from the work of Walsh, Coffey, and their group at Johns Hopkins; Grayhack, Lee, and the group at Northwestern; and Cunha and others. A complete discussion of prostate physiology and the pathophysiology of BPH can be found in Chapters 90 and 91 of Campbell-Walsh Urology, 10th edition (“Development, Molecular Biology, and Physiology of the Prostate” and “Benign Prostatic Hyperplasia: Etiology, Pathophysiology, Epidemiology, and Natural History”). There is no question that a functioning testis is a prerequisite for the normal development of the prostate in animals and humans. Males castrated before puberty do not develop BPH. BPH is rare in males castrated before the age of 40. Androgen deprivation in older men reduces prostate size. Patients with diseases that result in impaired androgenic production or metabolism have reduced or minimal prostatic growth. Although other endocrine factors are no doubt involved, the androgenic influence on prostatic growth and function is obviously central, although endocrine evaluation of the aging male has disclosed no recognizable surge in androgen secretion. The prostate develops from the urogenital sinus during the third fetal month under the influence of dihydrotestosterone (DHT) produced from fetal T via 5-alpha reductase. During development there is a close but incompletely understood interaction between the stromal and epithelial components. DHT is produced from T in the stroma cell and has an autocrine effect there and a paracrine effect in the epithelial cell. These effects are thought to include induction of multiple growth factors and alteration in the extracellular matrix. Prostate growth and maintenance of size and secretory function are stimulated by serum T, converted within the prostate to DHT, a compound whose relative androgenicity is higher. Free plasma T enters prostatic cells by diffusion and is rapidly metabolized to other steroids. More than 90% is irreversibly converted to the main prostatic androgen, DHT, by the enzyme 5-alpha reductase. DHT or T is bound to specific androgen receptors in the nucleus, when activation of the steroid receptor occurs. Originally, an abnormal accumulation of DHT in the prostate was hypothesized as a primary cause of BPH development. However, Coffey and Walsh showed that human BPH occurs in the presence of normal prostatic levels of DHT. Estrogen-androgen synergism has been postulated as necessary for prostatic growth, as have other steroid hormones and growth factors. Although much remains to be elucidated regarding the hormonal interactions and necessities for the induction and maintenance of BPH, it is clear that clinically a reduction in prostate size of approximately 20% to 30% can be induced by either interfering with androgen-receptor binding or metabolism. This theory, first introduced by Cunha and associates, postulates that a delicate stromal-epithelial balance exists in the prostate and that stroma may mediate the effects of androgen on the epithelial component, perhaps by the production of various growth factors and/or autocrine and paracrine messengers. This is attributed to Issacs and associates and hypothesizes that BPH may result from abnormal maturation and regulation of the cell renewal process. In simple terms, this postulates that abnormal size in an aged prostate is maintained not by the increase in the rate of cell replication but rather by a decrease in the rate of cell death. Hormonal factors, growth factors, and oncogenes all influence this balance of replication and cell death. The exact interaction of these and possibly other factors and what determines the setting points for the level of cells in the prostate and their rates of growth, replication, and death are of major importance in understanding both BPH and prostate cancer. It is extremely important to understand the concepts of the two prostatic components contributing to BOO caused by BPO. The static component is due to bulk and includes elements of the stromal and epithelial cells and extracellular matrix. Androgen ablation, at least in short-term studies, affects primarily the epithelial cell population volume. Long-term effects on stromal and matrix volume and effects on aspects of stroma and matrix other than volume have not been excluded, however. Therapeutic modalities that reduce the size of the prostate or “make a hole” or enlarge one are directed primarily toward this bulk component. The dynamic component of obstruction refers to the contribution of prostatic smooth muscle. The tension of prostatic smooth muscle is mediated by alpha-1 adrenergic receptors, most of which are in the prostatic stroma. Alpha-1 receptors also exist in the smooth muscle of the bladder neck and the prostatic capsule. Activation of these contractile receptors can occur either through circulating catecholamine levels or through adrenergic innervation. Prostatic intraurethral pressure can be reduced experimentally by as much as 40% after systemic administration of an alpha-receptor antagonist. This dynamic component may be responsible for the well-recognized variation in symptoms over time experienced by many patients and may account for exacerbation of symptoms experienced by some individuals in response to certain foods, beverages, change in temperature, and levels of stress. This two-component idea was first popularized by Marco Caine and later developed by Herb Lepor and Ellen Shapiro, resulting in the successful application of selective alpha-adrenergic blocking agents for the treatment of BPH symptoms. The ratio of stroma to epithelium in the normal prostate is approximately 2:1, and in BPH approximately 5:1. These data for BPH are derived primarily from small resected prostates; the ratio for larger glands with epithelial nodules may be lower. Although the smooth muscle content of stroma has not been precisely determined, a significant proportion of the stroma is in fact smooth muscle. LUTS is a rubric, introduced by Abrams, to replace the term prostatism, which implied that the prostate was responsible for most (or all) symptomatic voiding complaints in men. LUTS with its subdivisions, filling/storage symptoms and voiding/emptying symptoms, have replaced the terminology of irritative and obstructive symptoms, both rather imprecise terms that imply an etiology that may be incorrect. Voiding/emptying symptoms include impairment in the size and force of the urinary stream, hesitancy and/or straining to void, intermittent or interrupted flow, a sensation of incomplete emptying, and terminal dribbling, although the last by itself seems to have little clinical significance. Filling/storage symptoms include nocturia, daytime frequency, urgency, and urgency incontinence. Emptying/voiding symptoms are the most prevalent, but filling/storage symptoms are the most bothersome to the patient and interfere to the greatest extent with daily life activities. LUTS associated with BPE and BPO are, however, not simply solely due to BOO. Such symptoms are due, in varying proportions in different individuals, to obstruction, obstruction-related changes in detrusor structure and function, age-related changes in detrusor structure and function, and changes in neural circuitry that may occur secondary to these factors. 2. Bladder changes secondary to obstruction can occur. These consist of bladder wall thickening, trabeculations (which are also associated with involuntary bladder contractions), and bladder diverticula (which could also be congenital). Bladder calculi can develop. Bladder decompensation can occur, and gross bladder distention can result. Chronically increased residual urine volumes may result and may contribute to frequency and urgency and persistent urinary infection. Acute urinary retention may supervene. Azotemia may result from upper tract changes. There is an increased incidence of lower urinary tract infections (UTIs) in obstructed patients. 3. Upper tract changes of ureterectasis, hydroureter, and/or hydronephrosis can result. These can result either from secondary vesicoureteral reflux, sustained high-pressure bladder storage without reflux, and sustained high-pressure attempts at emptying. Ureteral obstruction could also occur secondary to muscular hypertrophy or angulation at the ureterovesical junction. Hematuria may arise from dilated veins coursing over the surface of the enlarged adenomatous prostate. The urodynamics of BOO are described in Chapter 14. Patients with BPO characteristically exhibit decreased mean and peak flow rates, an abnormal flow pattern characterized by a long, low plateau, and elevated detrusor pressures at the initiation of and during flow. They may or may not have residual urine. Approximately 50% of such patients are found to have detrusor overactivity during filling. Pressure-flow urodynamics testing (UDS) can demonstrate detrusor underactivity. In this circumstance, it usually cannot be determined whether obstruction exists or existed prior, accounting for the detrusor dysfunction — a major issue. Specialized variations of UDS, either with or without video, are often helpful to separate BPO from other forms of outlet obstruction (see Chapter 14). If the residual urine volume is significant, its reduction is important in the evaluation of results of treatment of BPO. For many with a significant residual volume, it is impossible to differentiate deficient bladder contractility from outlet obstruction as the primary cause, without a pressure-flow study. Most agree that a large residual urine volume reflects a degree of bladder dysfunction, but it is difficult to correlate residual urine with either specific symptomatology or other urodynamic abnormalities. The most popular noninvasive method of measurement is ultrasonography. The error for ultrasound has been estimated at 10% to 25% for bladder volumes greater than 100 mL and somewhat worse for smaller volumes. Residual urine volumes in an individual patient at different times can vary widely. Reflux and large diverticula may complicate the accuracy of measurement. Paul Abrams and colleagues, after a thorough review of the subject, concluded that elevated residual urine has a relation to prostatic obstruction, although not a strong one, as supported by the following observations. 1. Elevated residual urine is common in the elderly of both genders. 2. The absence of residual urine does not rule out severe obstruction. 3. Elevated residual urine does not have a significant prognostic factor for a good operative outcome. Volume of more than 300 mL may correlate with unfavorable outcome. What constitutes an abnormal residual urine? The International Consultation on BPH concluded that a range of 50 to 100 mL represents the lower threshold to define abnormal. There is discussion ongoing as to the concept that it may be more clinically meaningful to describe residual urine volume as a percent of bladder capacity rather than as an absolute number. Significant disagreement exists regarding what constitutes an adequate urodynamic evaluation of LUTS in the male and whether a urodynamically quantifiable definition of obstruction is necessary or desirable before beginning treatment. Of all these urodynamic studies, uroflowmetry seems to excite the least controversy. Although diminished flow may be caused by either outlet obstruction or impairment of detrusor contractility, and outlet obstruction may certainly exist in the presence of a normal flow, it is acknowledged that most men with BOO do have a diminished flow rate and altered flow pattern. What is a normal flow rate? Paul Abrams and Derek Griffiths originally proposed that, empirically, peak flow rates of less than 10 mL/sec were associated with obstruction, peak flow rates greater than 15 mL/sec were not associated with obstructed voiding, and peak flow rates between 10 and 15 mL/sec were equivocal. Although this proposal has been widely used, it is generally acknowledged that flow rates at any level may be associated with either obstruction or lack of obstruction. Studies are cited showing that 7% to 25% of patients referred with LUTS had high flow BOO. Potential problems related to uroflow include the following: 1. Many patients do not or will not void in a volume sufficient for accurate measurement. 2. Others void with an interrupted stream or with postvoid dribbling, which makes interpretation of the endpoint of micturition difficult, casting some element of subjectivity into the calculation of average flow rate. 3. Some patients are unable to relax sufficiently to void in the same manner in which they would in the privacy of their own bathroom. 4. A considerable discrepancy may exist between the first and subsequent measures of mean and peak flow. 5. The flow parameter measured noninvasively and in the course of pressure flow studies in the same individual may vary considerably. Flow data changes can be expressed in terms of absolute change, percent change, or as cumulative frequency distribution. Clearly important is the initial flow number, the value of which may make the absolute or percent change look better or worse. In other words, raw data must be expressed along with the other frills that may be added to embellish flow data. It should be noted also that it is unknown what change in flow is necessary to give the impression of mild, moderate, or marked improvement. Because voiding events may be different from point to point in an individual’s life, a variety of flow nomograms have been constructed to facilitate comparison of them. It should be noted that many nomograms and tables of “acceptable flow rates” are available for various age groups. Many believe that the Siroky nomogram, commonly used in the United States, overestimates peak and average flow rates for older men and therefore underestimates the number of older men with BOO. Other nomograms include the Drach peak-flow nomogram and the Liverpool and Bristol nomograms. It is doubtful that consistency will be achieved among flow nomogram makers. However, one of the systems supported by at least a portion of urodynamicists should be utilized for comparison following treatment of BPO. Filling cystometry provides information on sensation, compliance, and the presence of and threshold for involuntary bladder contractions and urodynamic bladder capacity. Compliance is generally not affected in patients with BPO, but as mentioned previously, approximately 50% of such patients will have involuntary bladder contractions. On a logical basis, BOO would seem to be defined by the relationship between flow rate and detrusor contractility. Outlet obstruction is best characterized by a poor flow rate in the presence of a detrusor contraction of adequate force, duration, and speed. With obstruction, detrusor pressure during attempted voiding generally rises, flow rates generally fall, and the shape of the flow curve becomes more like a plateau than a parabola.
Benign Prostatic Hyperplasia and Related Entities
General considerations
Definitions and epidemiology
Prostatic size and morphology pertinent to BPH
Etiologic theories of BPH: pathophysiology
Hormones
Stromal-epithelial interaction theory
Stem-cell theory
Static and dynamic components of prostatic obstruction
Lower urinary tract symptoms
Signs of BPH
Urodynamics of BOO
Residual urine volume
Uroflowmetry
Cystometry and pressure-flow studies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


