Chapter 79A Benign liver lesions
Overview
One of the consequences of extensive use of imaging for an ever-increasing number of indications is the detection of asymptomatic tumors. In the absence of underlying chronic liver disease or a history of cancer, the vast majority of these lesions correspond to benign liver tumors, including cystic and solid lesions. Cystic tumors will be covered in Chapter69A, 69B . Solid benign lesions include a broad spectrum of regenerative and true neoplastic processes (Table 79A.1). Based on the cell of origin, most solid, benign tumors can be classified into two groups: epithelial lesions include hepatocellular (focal nodular hyperplasia [FNH] and adenoma) and cholangiocellular tumors (bile duct adenoma); mesenchymal tumors include those lesions that originate from blood vessels (hemangioma), adipose tissue (angiolipoma), and muscle (leiomyoma). Except hepatocellular adenoma (HA), which may be associated with serious complications that may require surgery, the vast majority of solid, benign liver lesions remain asymptomatic and do not increase in volume; therefore they do not require any treatment or follow-up.
Table 79A.1 Histologic Classification of Benign Liver Lesions and Main Clinical Data
| Lesion | Type | Comments |
|---|---|---|
| Epithelial Lesions | ||
| Hepatocytes | Hepatocellular adenoma | |
| Focal nodular hyperplasia | ||
| Nodular regenerative hyperplasia | ||
| Focal fatty change | ||
| Biliary cells | Bile duct adenoma | |
| Biliary hamartoma (Von Meyenburg complex) | ||
| Nonepithelial Lesions | ||
| Mesenchymal | Hemangioma | |
| Angiomyolipoma | ||
| Lipoma, myelolipoma | ||
| Heterotopia | Adrenal, pancreatic, or spleen tissues | |
| Others | Peliosis hepatis | |
| Inflammatory pseudotumor | ||
The understanding of clinical, biologic, radiologic, and pathologic characteristics of each tumor is important for accurate diagnosis and appropriate management. Advances in imaging studies allow an accurate diagnosis in the majority of cases, reducing the use of percutaneous biopsy to only a few patients and limiting the number of resections for final diagnosis to exceptional cases. The most frequent benign liver cell lesions include hemangioma, FNH, and HA. Because radiologic imaging is of critical importance in diagnosis and management, there is considerable emphasis on the role of radiologic study, and this section should be read in conjunction with Chapters 13, 16, and 17.
Cavernous Hemangioma
Hepatic hemangiomas are probably the most common of all liver tumors. These tumors occur at all ages, however, because of the difference of histologic structure between the adult and the infantile forms (see Chapter 82) and the different clinical presentation, they must be regarded as separate entities (Hobbs, 1990). Only hemangioma of the liver in adults will be considered in this chapter.
Pathogenesis and Pathology
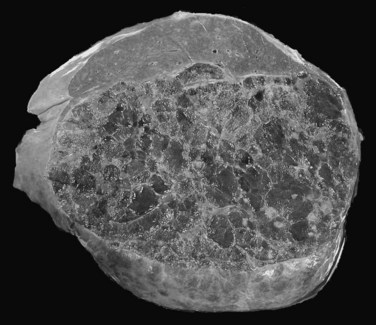
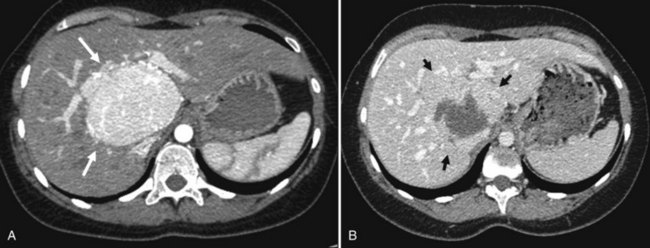
The prevalence of hemangioma in the general population ranges from 1% to 20% (Semelka & Sofka, 1997), but it is predominant in women by a 5 : 1 ratio (Mergo & Ros, 1998; Trotter & Everson 2001; Biecker et al, 2003). In adults, hemangiomas are usually found in patients at a mean age of 50 years and equally in the left and right lobes of the liver; the majority are less than 5 cm in diameter and are rarely pediculated, but hemangiomas measuring 10 cm or more are referred to as giant hemangiomas and can present with fibrosis, thrombosis, and calcifications (Fig. 79A.1).
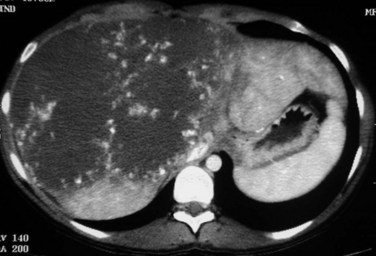
FIGURE 79A.1 Giant hemangioma. Portal-venous phase helical computed tomographic and centripetal contrast enhancement.
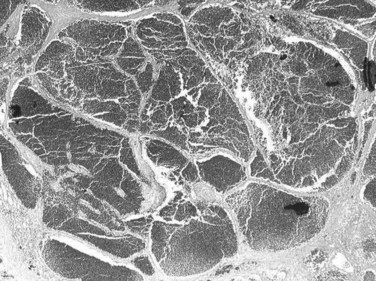
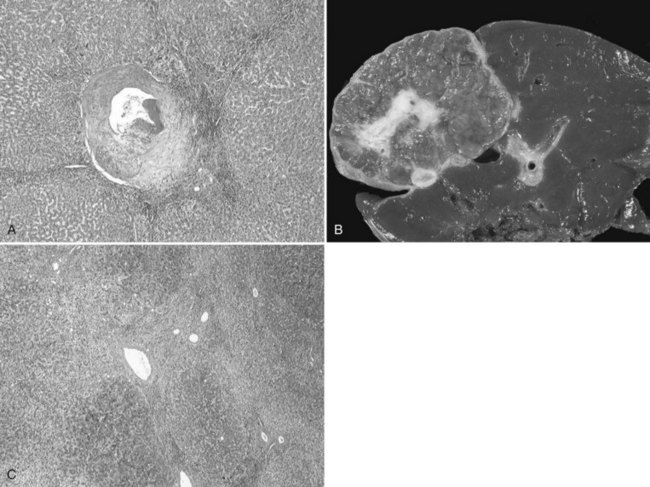
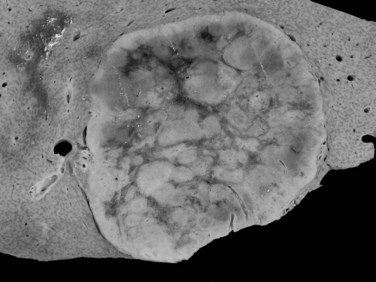
The pathogenesis of hemangioma is not well understood. Some of these tumors have estrogen receptors, and accelerated growth has been observed with high-estrogen states, such as those associated with puberty, pregnancy, oral contraceptive use, and with androgen treatment. These findings suggest that hormonal effect may be one of the pathogenic mechanisms (Trotter & Everson, 2001; Cobey & Salem, 2004). Macroscopic examination demonstrates well-delineated, flat lesions of red-blue color that may partially collapse on sectioning. Some degree of fibrosis, calcification, and thrombosis may be observed, most commonly in the largest lesions (Fig. 79A.2). Microscopically, hemangiomas are made of cavernous vascular spaces lined by flattened endothelium underlying fibrous septa of various widths (Fig. 79A.3). Small hemangiomas may become entirely fibrous, appearing as a solitary fibrous nodule.
Clinical and Biologic Data
The vast majority of hemangiomas are less than 5 cm and are found incidentally during ultrasound (US) or computed tomography (CT) examination of the abdomen for unrelated reasons (Trotter & Everson, 2001). Hemangiomas remain stable in size (Takayasu et al, 1990) or demonstrate minimal increase in diameter over time (Nghiem et al, 1997). Pain related to an uncomplicated hemangioma is most probably due to associated disorders, such as gallbladder disease, liver cysts, gastroduodenal ulcers, or a hiatal hernia (Farges et al, 1995). Large hemangiomas may be asymptomatic or may manifest with an abdominal mass or pain (Erdogan et al, 2007). Large lesions located in the left lobe of the liver may cause pressure effects on adjacent structures with resulting symptoms. Jaundice as a result of compression of bile ducts by hemangioma has also been observed, but this is rare (Losanoff & Millis, 2008).
Some cases of inflammatory processes complicating giant hemangioma have been reported (Bornman et al, 1987; Takayasu et al, 1990), and the prevalence is probably underestimated. Signs and symptoms of an inflammatory process include low-grade fever, weight loss, abdominal pain, accelerated erythrocyte sedimentation rate, normal white blood cell count, anemia, thrombocytosis, and increased fibrinogen level. The imaging features are those of giant hemangioma. Clinical and laboratory abnormalities disappear after surgical excision of the mass (Bornman et al, 1987; Pateron et al, 1991; Pol et al, 1998).
Kasabach-Merritt syndrome is an exceptional complication of hepatic hemangioma in adults (Hall, 2001; see Chapter 82). This coagulopathy consists of intravascular coagulation, clotting, and fibrinolysis within the hemangioma, and it may progress to secondary increased systemic fibrinolysis and thrombocytopenia (Concejero et al, 2009); the syndrome is reversible after removal of the hemangioma. Although rarely encountered in hepatic hemangioma, intratumoral hemorrhage can occur spontaneously or after anticoagulation therapy. Spontaneous rupture of a hemangioma is exceptional, and very few indisputable cases have been reported (Corigliano et al, 2003); taking into account the high prevalence of these tumors, the extremely low incidence of this complication should not interfere with the therapeutic management, nor should it influence the specific advice to patients, who are often worried by the presence of a vascular liver tumor (Plackett & Lin-Hurtubise, 2008).
Imaging
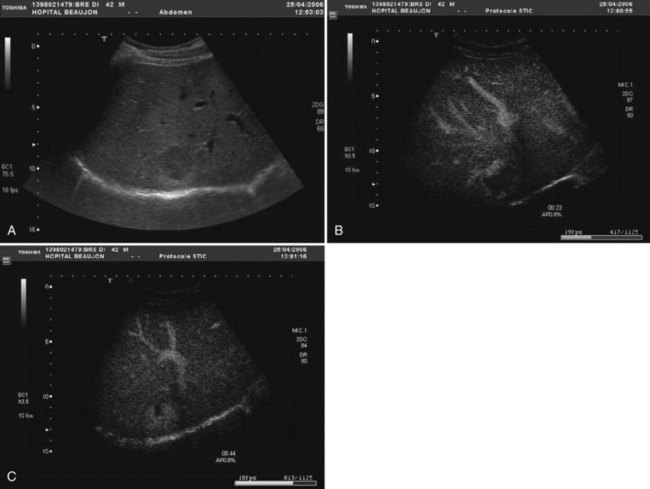
The vast majority of hemangiomas are diagnosed accurately by imaging studies alone because of characteristic imaging features. On US the classic appearance of hemangioma is that of a homogeneous hyperechoic mass less than 3 cm in diameter with acoustic enhancement and sharp margins (Fig. 79A.4; see Chapter 13). No vascular pattern is usually identified on color Doppler. Other investigations are required when US does not show typical patterns. Contrast-enhanced US reveals peripheral globular enhancement in the portal phase and isoechoic pattern on the late phase in most atypical hemangiomas (Quaia et al, 2002).
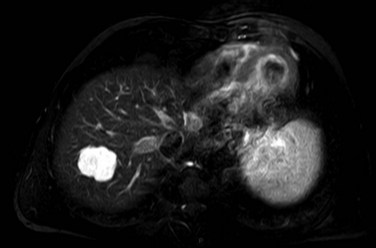
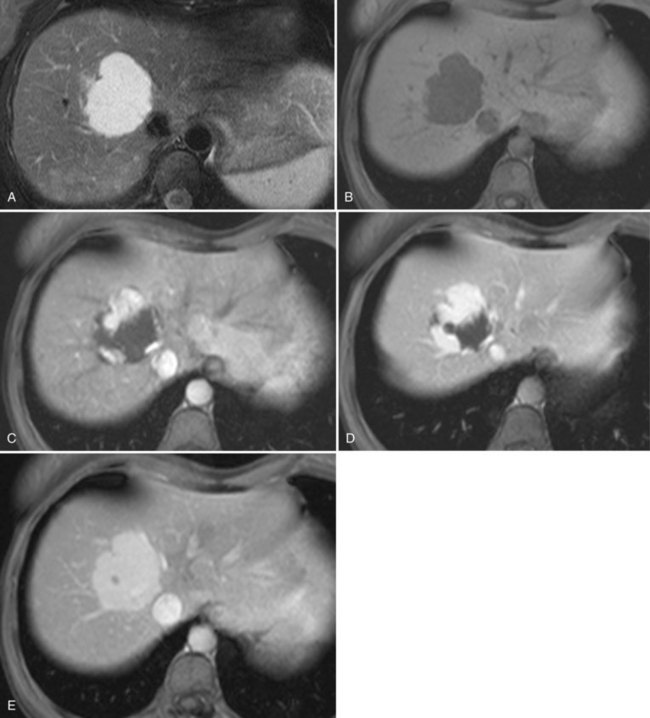
On CT, the criteria for the diagnosis of hemangioma are the following: 1) low attenuation on noncontrast CT, 2) peripheral and globular enhancement of the lesion followed by a central enhancement on contrast CT, and 3) contrast enhancement of the lesion on delayed scans (Freeny & Marks, 1986; see Chapter 16). Among these criteria, the presence of peripheral puddles on the arterial phase has a sensitivity of 67%, a specificity of 99%, and a positive predictive value of 86% for hemangioma (Nino-Murcia et al, 2000). Magnetic resonance (MR) imaging is the key imaging modality in the characterization of liver hemangiomas (Itai et al, 1985; Starck et al, 1985; see Chapter 17). The classic appearance of a liver hemangioma is that of a hypointense lesion on T1-weighted sequences, a strongly hyperintense lesion on heavily T2-weighted sequences, with a “lightbulb” pattern (Fig. 79A.5). Dynamic multiphasic T1-weighted sequences after gadolinium chelate administration show findings similar to that of contrast-enhanced CT phases (Fig. 79A.6; Semelka et al, 1994). Hemangiomas can be multiple in 10% of cases, and in exceptional cases, the presence of innumerable hemangiomas are called hemangiomatosis (Ishak & Rabin, 1975; Keegan et al, 2001).
The two most common imaging atypias are found in giant hemangiomas and in rapidly filling hemangiomas. Giant hemangiomas, which are defined when they exceed 6 or 12 cm in diameter, are often heterogeneous with marked central areas that correspond to thrombosis, extensive hyalinization, and fibrosis (Danet et al 2003; Coumbaras et al, 2002). However, usually the typical early, peripheral, nodular enhancement is observed along with strong hyperintensity on T2-weighted MR images at the periphery. Although present, the progressive centripetal enhancement of the lesion does not lead to complete filling. Rapidly filling hemangiomas are not uncommon and occur significantly more often in small lesions (42% of hemangiomas are <2 cm in diameter) (Hanafusa et al, 1995). CT and MR imaging show an immediate homogeneous enhancement in the arterial phase, which makes differentiation from other hypervascular tumors difficult. Their diagnosis is based on strong hyperintensity on T2-weighted images, the parallel enhancement with arterial structures, and the persistent enhancement on delayed-phase imaging; it is important to note that these rapidly filling hemangiomas may induce arterial–portal venous shunts (Kim et al, 2001). The other atypical hemangiomas are very uncommon and include very slow filling, calcified, hyalinized cystic and pedunculated hemangiomas, those with fluid-fluid levels, and hemangiomas with capsular retraction.
Hemangiomas associated with inflammatory response syndrome are usually due to acute thrombosis of a part or all the hemangioma. These can be diagnosed by showing spontaneous hyperattenuation on unenhanced CT scans. Hemangiomas developing in abnormal liver are difficult to diagnose, and those in fatty liver usually appear isoechoic or hypoechoic at US and hyperattenuating at unenhanced CT (Marsh et al, 1989). MR imaging with fat-suppressed, in-phase and opposed-phase T1-weighted sequences is the key to the diagnosis and is more helpful than CT; strong hyperintensity on T2-weighted images is also a reliable finding.
With progressive cirrhosis, hemangiomas are likely to decrease in size and become more fibrotic and difficult to diagnose radiologically (Brancatelli et al, 2001). In this clinical setting, US cannot be used to confidently make the diagnosis of hemangioma, as half of the hyperechoic lesions are hepatocellular carcinomas (HCCs) (Caturelli et al, 2001). The association of hepatic hemangioma and FNH can be observed in about 25% of cases and is not fortuitous (Mathieu et al, 1989; Vilgrain et al, 2003). FNH is considered to be a hyperplastic response as a result of focal increased arterial flow in the hepatic parenchyma and, like hemangioma, it is thought to have a vascular origin.
Imaging, and especially MR imaging, is therefore able to diagnose liver hemangiomas in nearly all cases, and liver biopsy should be restricted to exceptional cases. Liver hemangioma, which has been considered a contraindication to needle biopsy for many years, is possible without significant risk of hemorrhage (Heilo & Stenwig, 1997; Caldironi et al, 1998). However, as in other tumors, a cuff of normal hepatic parenchyma should be interposed between the capsule and the margin of the hemangioma.
Management
Indications for treatment include severe symptoms, complications, and inability to exclude malignancy (Yoon et al, 2003; Duxbury & Garden, 2010). Symptoms are more frequently encountered with huge hemangioma (Erdogan et al, 2007). Surgical resection remains the definitive treatment, but other less effective options include hepatic artery ligation and radiation therapy; radiation therapy can reduce the size of the lesion (Gaspar et al, 1993), but long-term effects of radiation therapy on the liver and adjacent structures can be deleterious. In addition, relief of symptoms is not well documented. Arterial embolization, which may be considered for the temporary control of hemorrhage, has limited success and is occasionally associated with morbidity (Reading et al, 1988). Arterial ligation may be considered during surgical procedures to allow manual decompression of large hemangiomas and facilitate their manipulation and enucleation (Yoon et al, 2003).
When indicated, the only treatment to be considered is resection (Herman et al, 2005; Erdogan et al, 2007), and it should be performed in specialized units, where a variety of techniques and approaches can be used. The choice between enucleation and resection requires consideration of the size and anatomic location of the lesion. Hemangioma located in the peripheral liver area is preferably treated by enucleation, whereas tumors deeply located are more safely resected with a formal anatomic liver resection (Kuo et al, 1994; Gedaly et al, 1999; Fu et al, 2009). In the majority of cases, patients showed symptoms relief after resection of a symptomatic hemangioma (Erdogan et al, 2007). Although laparoscopic resection is safe for peripheral hemangioma (Karahasanoglu et al, 2001; Jiang et al, 2007), this approach should not extend the rare indication for surgery. Liver transplantation has also been used successfully to treat symptomatic patients with a technically unresectable, complicated giant hemangioma or hemangiomatosis with cardiopulmonary complications (Browers et al, 1997; Tapetes et al, 1995; Ferraz et al, 2004; Keegan et al, 2001; Ercolani et al, 2010).
Focal Nodular Hyperplasia
Pathogenesis and Pathology
FNH accounts for the second most common benign liver process, after hemangioma. It is a benign, tumor-like condition predominantly diagnosed in women 30 to 50 years of age, and it is not influenced by oral contraceptives (International Working Party, 1995; Ishak, 1979). FNH is considered a hyperplastic reaction resulting from arterial malformation (Wanless et al, 1985). This hypothesis, suggesting that increased arterial flow hyperperfuses the local liver parenchyma and leads to secondary hepatocellular hyperplasia, has been reinforced by molecular data showing that FNH is a polyclonal regenerative process (Gaffey et al, 1996; Paradis et al, 1997; Rebouissou et al, 2008). This regenerative process induced in a specific vascular territory could explain the absence of size changes in the vast majority of cases. However, an involution of this lesion is possible after menopause (Kuo et al, 2009), which explains the occurrence of this lesion in patients with vascular disorders of the liver, including Budd-Chiari syndrome (Cazals-Hatem et al, 2003), hereditary hemorrhagic telangiectasia (Gincul et al, 2008), congenital absence of portal flow (Kim T, et al, 2004), or portal thrombosis with subsequent hepatic arterialization (Bureau et al, 2004).
Such focal regenerative processes are also described in cirrhotic tissue with the so-called FNH-like macronodules. FNH displays a typical pathologic pattern for both radiologists and pathologists. Grossly, it is a well-circumscribed, unencapsulated, usually solitary mass characterized by a central fibrous scar that radiates into the liver parenchyma (Fig. 79A.7). Histologically, FNH is composed of benign-appearing hepatocytes arranged in nodules, usually partially delineated by fibrous septa that originate from the central scar. The main diagnostic feature is the presence of large and dystrophic vessels in the fibrous septa, which can be accompanied by several degrees of ductular proliferation and inflammatory cells. The hepatocytes are hyperplastic, arranged in liver plates of normal or slightly increased thickness (see Chapter 78). Hepatocytes may be hydropic, related to cholestatic changes, or they may display some degree of steatosis.
Performance of biopsy in the diagnosis of FNH is often a challenge, because fibrous septa and thick abnormal arteries are usually missing on biopsy specimen (Makhlouf et al, 2005). In that context, glutamine synthetase showing focal positive hepatocellular areas, usually centered by hepatic veins and described as a maplike pattern, is a helpful surrogate marker (Bioulac-Sage et al, 2009a).
Besides this classic form of FNH, several variant lesions are described with increased frequency and commonly classified as nontypical FNH by radiologists. This group of lesions is somewhat heterogeneous, including FNH without a central fibrous scar and FNH containing fat (Nguyen et al, 1999). However, molecular studies have demonstrated that in this group of atypical FNH, lesions that display telangiectatic changes, the so-called telangiectatic form of FNH, are clonal processes that should be rather regarded as a variant form of liver cell adenoma; it is classified as a specific subgroup of adenoma other than FNH (Paradis et al, 2004; Bioulac-Sage et al, 2005).
Clinical and Biologic Data
FNH represents the second most frequent benign lesion, with an estimated prevalence around 1% (Vilgrain, 2006); it is seen predominantly in women (9 : 1) and often is discovered in patients aged 30 to 40 years, although men with FNH are usually older than 40 years (Nguyen et al, 1999). The vast majority of FNH is asymptomatic, usually discovered incidentally during liver US examination. In a few patients with large tumors, FNH can be discovered by abdominal pain or discomfort. Large lesions located in the left lobe of the liver may cause pressure effects on adjacent structures with resulting symptoms. The lesion may be felt when it is pedunculated, and it can be responsible for acute episodes of pain because of torsion on the pedicle. Large FNH below the Glisson capsule could also be responsible for pain (Buetow et al, 1996), but in most cases pain related to FNH is probably due to associated disorders.
Complications of FNH, such as rupture or bleeding, are exceptional and are usually related to atypical forms of FNH with specific imaging and biologic features, the so-called telangiectatic FNH, which should be classified as an adenoma. No malignant transformation of FNH has been demonstrated. A reduction in size after menopause may be observed, but in the vast majority of cases, FNH remains stable in size, even after interruption of oral contraception or after pregnancy (Mathieu et al, 2000).
Liver biologic test results are normal in nearly 80% of cases (Belghiti et al, 1993). Abnormalities include mild elevation of GGT and alkaline phosphatase in patients with a large FNH that caused extrinsic compression of intrahepatic biliary ducts. The presence of a slight elevation of serum transaminase levels could be due to the presence of associated steatosis in the underlying liver parenchyma.
Imaging
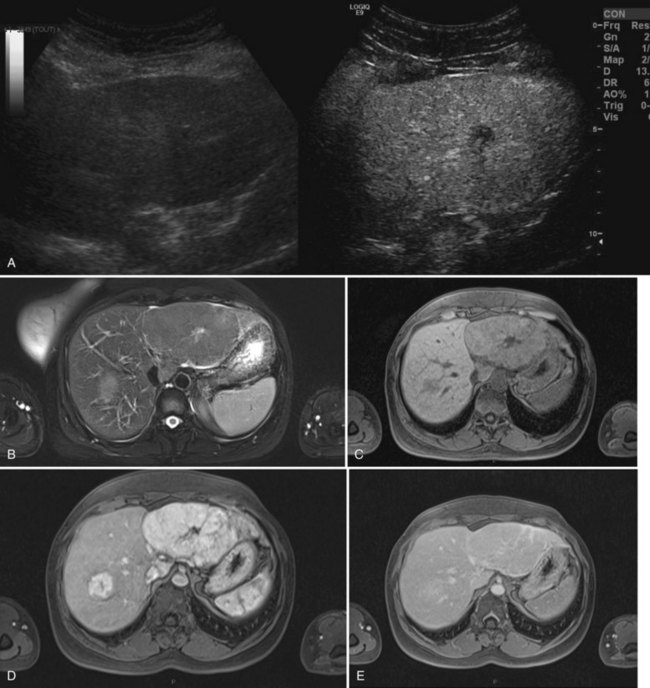
FNH is usually slightly hypoechoic or isoechoic at US (see Chapter 13). Some lesions are only detected because they displace the surrounding vessels. Lobulated contours and a hypoechoic halo are often observed; the central scar is slightly hyperechoic but is often difficult to visualize at US (20% of cases; Shamsi et al, 1993). Typical findings at color Doppler include the presence of a central feeding artery with a stellate or spoke-wheel pattern that corresponds to the artery running from the central scar to fibrous septa (Fig. 79A.8). Doppler spectral analysis shows, in most cases, an intralesional pulsatile waveform with high diastolic flow and low resistive index (Uggowitzer et al, 1997). Large draining vessels may be identified in the periphery at the tumor margins. US contrast agents and nonlinear continuous imaging modes at a low mechanical index are very interesting tools to better characterize FNH. Most lesions enhance at the arterial phase (Kim MJ, et al, 2004; Dietrich et al, 2005) with a central vascular supply and a centrifugal filling to the periphery. The lesion becomes homogeneously isoechoic in the late portal and delayed phases in the vast majority of the cases (Kim MJ, et al, 2004), and very little FNH is evident with washout; when the central scar is detected, it appears hypoechoic on both arterial and portal phases. By showing centrifugal enhancement with radiated vascularization, differentiation from hepatocellular adenoma is possible in most cases (Kim T, et al, 2008).
On precontrast CT scans, FNH is demonstrated as a focal hypodense or isodense mass. A central hypodense scar is depicted in only one third of the patients (Shamsi et al, 1993). Calcifications within the central scar are very rare and are observed in only about 1% (Caseiro-Alves et al, 1996). Because of the prominent arterial supply to the FNH, the lesion enhances rapidly at the arterial phase of contrast-enhanced CT in most cases (Brancatelli, et al 2001). Lesion enhancement decreases at the portal venous phase, and the lesion may be either isodense or slightly hyperdense relative to normal liver. Additional findings are lesion homogeneity, a lobulated contour, and the absence of a capsule. A central scar is observed more often in large lesions than in small ones, and it enhances over time (Carlson et al, 2000). It has been shown that the enhancement of FNH in the arterial phase is significantly higher than in HA (Ruppert-Kohlmayr et al, 2001).
On MRI, typical FNH is isointense or hypointense on T1-weighted images and isointense or slightly hyperintense on T2-weighted images (Vilgrain et al, 1992; Buetow et al, 1996; see Chapter 17). The central scar is hypointense on T1-weighted images and strongly hyperintense on T2-weighted images (Mortelé et al, 2000; Kehagias et al, 2001). Another key finding is the lesion homogeneity apart from the central scar. After administration of gadolinium chelates, the enhancement is similar to that observed on contrast-enhanced CT. On delayed-phase imaging, 5 to 10 minutes after injection, the central scar shows high signal intensity because of the accumulation of contrast material in the fibrous tissue. Hepatobiliary contrast agents can be used to highlight the hepatocellular origin of the lesions and to differentiate FNH from hepatocellular adenomas; however, the biokinetics are different among those specific contrast agents (Ba-Ssalamah et al, 2002; Grazioli et al, 2005). On diffusion-weighted MR sequences, FNH is often hyperintense on high b values, but their apparent diffusion coefficient (ADC) values are close to that of the liver.
Atypical forms of FNH that are difficult to diagnose include FNH without a scar, especially in lesions measuring less than 3 cm in diameter. In some cases, scars do not enhance on delayed imaging, or they are hypointense on T2-weighted images; this leads the clinician to suspect the diagnosis of fibrolamellar carcinoma. Other atypical findings include hyperintensity on T1-weighted imaging, which may be caused by various pathologic changes that include fat deposition (mostly in patients with steatosis; Stanley et al, 2002). FNH heterogeneity is exceptional and should make the diagnosis doubtful.
Although FNH is mainly a solitary lesion, multiple FNH lesions may be observed in 20% of cases and may be associated with hemangiomas or more seldom with HA (Mathieu et al, 1989; Vilgrain et al, 2003; Laurent et al, 2003). Accuracy of contrast-enhanced US has been established, and MR imaging has the higher sensitivity (70%) and specificity (98%) (Soussan et al, 2010). However, the diagnosis of FNH is based on a combination of features, and none of them are specific for FNH. When imaging cannot establish a firm diagnosis, liver biopsy plays a role in diagnosing FNH (Fabre et al, 2002; Makhlouf et al, 2005).
Management
Surgical resection is restricted to symptomatic patients. Symptomatic patients with confirmed FNH should be thoroughly investigated to exclude other pathology before attributing symptoms to the liver lesion. The development of a liver laparoscopic approach should not extend the indication for resection, which should be performed in specialist hepatobiliary units, as it is clearly indicated in patients with large, symptomatic FNH located in the left liver and in those with a pedunculated lesion. The safer resection procedure is liver resection with a surgical margin, rather than enucleation, because large veins often surround FNH, which renders enucleation difficult. In some symptomatic patients with large FNH located in the right liver or in segment I and necessitating difficult or risky resection, transarterial embolization has been advocated to determine the relationship between the lesion and symptoms (De Rave & Hussain, 2002; Kehagias et al, 2001). Data in the literature (Amesur et al, 2009) and our experience have shown that this is an attractive treatment with reduction of size in conjunction with relief of symptoms (Fig. 79A.9). Percutaneous radiofrequency ablation (RFA) has also been described as an effective treatment modality for symptomatic FNH (Hedayati et al, 2010).
Hepatocellular Adenoma
Pathogenesis and Pathology
Hepatocellular adenoma (HA) is a rare, benign liver neoplasm strongly associated with oral contraceptive (OC) use (Rosenberg, 1991). The most important complications of HA are hemorrhage and malignant transformation into HCC. HA is mainly observed in women of childbearing age, and it is rare in men (sex ratio 9 : 1). Its incidence is estimated to be 0.1 per year per 100,000 in non-OC users, and it reaches 3 to 4 per 100,000 in long-term OC users (Wittekind, 1995). HA is directly related to OC use, and the risk for developing adenoma increases with the duration and estrogen content of the medication (Rosenberg, 1991; Dokmak et al, 2009). This tumor can also occur in men receiving anabolic steroids (Socas, et al 2005), or it may be associated with underlying metabolic disease, including type 1 glycogen storage disease, and iron overload related to β-thalassemia and hemochromatosis (Barthelemes & Tait, 2005; Bioulac-Sage et al, 2008). The prevalence of obesity was high in patients with HA (Dokmak et al, 2009), and it was recently described in patients with nonalcoholic fatty liver disease (Watkins et al, 2009). HA can also occur in patients with portacaval shunt or portal deprivation, and familial cases have been reported in patients with mellitus onset diabetes type 3 (Chiche et al, 2000; Bioulac-Sage et al, 2008).
HA is usually solitary, sometimes pedunculated, with a diameter that can vary from a few millimeters up to 30 cm. The widespread use and technical progress in imaging techniques has revealed that one third of patients have multiple HAs (Dokmak et al, 2009; Deneve et al, 2009; Bioulac-Sage et al, 2009b). Patients with more than 10 HAs are subclassified as having liver adenomatosis, considered to be more common in men and to have an increased risk of complications (Fléjou et al, 1985). However, review of the literature and our experience do not support this arbitrary classification based on clinical and imaging characteristics (Ribeiro et al, 1998; Jovine et al, 2004; Barthelemes & Tait, 2005; Dokmak et al, 2009; Bioulac-Sage et al, 2008). The risk of bleeding and of malignant transformation is similar to that in patients with solitary adenoma and is mainly related to the size and subtype of tumors (Dokmak et al, 2009; Bioulac-Sage et al, 2008; Deneve et al, 2009; Stoot et al, 2010). It also appears that the prevalence of HA is rising in men, mainly as a result of the rising incidence of metabolic syndrome (Farges et al, 2011). Such HA displays a significantly higher risk of malignant transformation to HCC, reaching 47% in the last few years in our experience (Farges et al, 2011); therefore patients should not be managed according to the number of HAs, but rather according to the risk of complications, which is related to sex, the histologic subtype, and mainly to the size of the HA.
Large subcapsular vessels are commonly found on macroscopic examination. On cut sections, the tumor is well delineated, sometimes encapsulated, and has a fleshy appearance; it ranges in color from white to brown (Fig. 79A.10). HA frequently displays heterogeneous areas of necrosis and hemorrhage. Histologically HA consists of a proliferation of benign hepatocytes arranged in a trabecular pattern; however, the normal liver architecture organization is absent (Chapter 78). Hepatocytes may have intracellular fat or increased glycogen. Malignant transformation to HCC may be difficult to assess, especially on biopsy specimen (Deneve et al, 2009; Stoot et al, 2010), but it is observed in 4% to 10% of cases in published series (Barthelemes & Tait, 2005; Bioulac-Sage, 2008; Deneve et al, 2009; Zucman-Rossi et al, 2006).