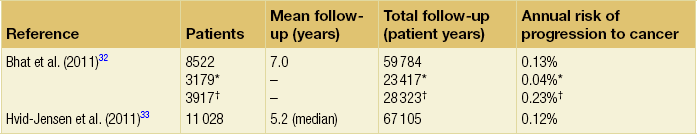
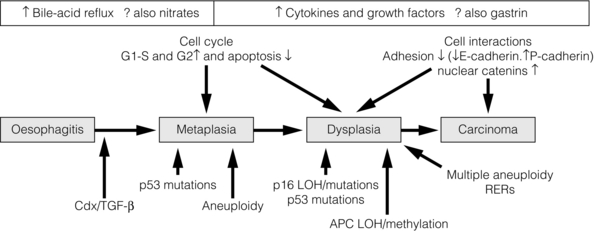
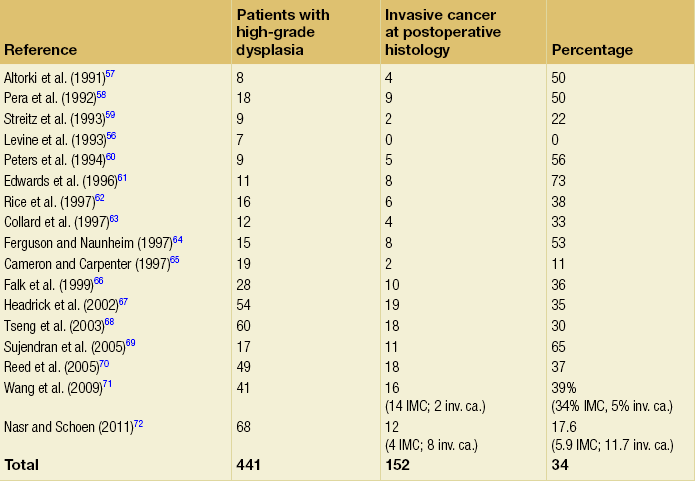
15 Barrett’s oesophagus is a change in any portion of the normal squamous oesophageal epithelium to a metaplastic columnar epithelium that is visible endoscopically and can be confirmed or corroborated histologically.1,2 There are three histologically distinct types of columnar metaplasia: intestinal (IM), cardiac (CM) and fundic. In the USA, unlike the UK and Japan, the diagnosis of Barrett’s oesophagus requires the identification of intestinalisation characterised by the presence of goblet cells. However, the UK definition considers that Barrett’s oesophagus is analogous to a ‘columnar lined oesophagus’ and does not require identification of goblet cells due to fears that sampling bias could lead to under-diagnosis and potentially exclude patients from surveillance programmes. It has been reported that a minimum of eight biopsies are required to confidently exclude intestinal metaplasia – if only four biopsies are taken the diagnostic yield is only 35%.3 The exact population prevalence of Barrett’s oesophagus is unclear. Data described in post-mortem and endoscopic series range from 0.9% to 5.6% depending on the precise definition used and the type of study.4–7 It is likely that the true prevalence in the West is around 2%. When extrapolated to the UK and US populations, conservative estimates of prevalence are 1 million and 4 million affected individuals, respectively.8 There is also some evidence that the incidence of Barrett’s oesophagus in the West is increasing by up to 2% per year.5,9–11 Data from the Netherlands demonstrated an increase in the number of cases of Barrett’s oesophagus despite a decrease in the number of endoscopies being performed over the same period, suggesting a true increase in incidence.11 The incidence of Barrett’s oesophagus increases with age, the mean age at diagnosis being approximately 62 years for men and 68 years for women. It predominantly affects Caucasians12 and is more common in men than women, with a ratio of approximately 1.7:1.13 The risk of developing Barrett’s is related to increased frequency and duration of reflux symptoms.14 This appears to correlate with the well-known association between increased frequency, duration and severity of reflux symptoms, and increased risk of adenocarcinoma of the oesophagus. The incidence of Barrett’s oesophagus in patients with symptomatic gastro-oesophageal reflux disease (GORD) is between 5% and 12%.9,15 Evidence from one case series suggests that more than 60% of patients with Barrett’s oesophagus develop the condition secondary to chronic GORD, although other causes of oesophagitis, including non-steroidal anti-inflammatory drugs (NSAIDs), chemotherapy and viral infections are also associated with the disease. It does raise an intriguing possibility that a smaller proportion of patients can develop Barrett’s de novo in the absence of obvious symptomatic or perhaps even pathological reflux. Therefore, other factors that may catalyse changes at the oesophagogastric junction (OGJ) are obesity and cigarette smoking,which have been identified as risk factors for both Barrett’s oesophagus and progression to malignancy.16 A Swedish case–control study demonstrated that patients with recurrent reflux symptoms, when compared with asymptomatic patients, had an odds ratio of 7.7 for oesophageal adenocarcinoma and 2.0 for adenocarcinoma of the gastric cardia. Patients with severe long-standing symptoms had an odds ratio of 43.5 and 4.4 for oesophageal and cardia adenocarcinoma, respectively.17 It is crucial to make a thorough and systematic inspection of the mucosa in order to identify any macroscopic neoplastic disease. Water or 1% acetylcysteine should be used to remove blood, saliva and refluxate from the oesophagus, and sufficient insufflation should be ensured to clearly visualise any mucosal abnormalities. Particular care must be taken to identify the OGJ in patients with a hiatus hernia as it is easy to miss the distal extent of a Barrett’s segment in these patients. Clinicians should be aware that at endoscopic inspection most areas of early neoplasia and cancer are detected in an area around the 2 to 4 o’clock position in the endoscopist’s view.19 This rigorous biopsy protocol, which is often poorly adhered to outside of specialist centres, samples less than 5% of the mucosa and may miss up to 57% of dysplasia.20,21 Advanced endoscopic imaging techniques may allow targeted biopsies from high-risk areas, improving diagnostic yield (Table 15.1).22–26 Potentially, these imaging tools may also facilitate targeted endoscopic resection of high-grade dysplasia (HGD) and intramucosal cancer. Table 15.1 Advanced endoscopic imaging modalities being investigated for use in Barrett’s oesophagus surveillance programmes and for facilitation of targeted endoscopic resection It is currently believed that Barrett’s metaplasia develops as a mucosal ‘adaptive’ response to increased cell loss as a result of chronic inflammation, secondary to GORD. Oesophageal squamous epithelium is highly sensitive to acid, alkaline and biliary reflux, which all cause inflammation, with cell loss, necrosis and ulceration. There is strong evidence that the site of origin of Barrett’s metaplasia is a progenitor stem cell located in the submucosal oesophageal gland ducts, following demonstration that a p16 point mutation originating in microdissected squamous duct tissue was also present in adjoining metaplastic crypts.27 Duodenal and gastric reflux-induced ulceration and inflammation is believed to induce tumour suppressor gene mutations, typically p53 and p16, in some of the stem cell populations located in oesophageal gland squamous ducts, which are present throughout the entire length of the oesophagus. Following this initiation phase, multiple distinct clones of metaplastic tissue compete to colonise the oesophagus, creating a mosaic pattern of clones across the segment. Clonal expansion of populations with greater selective advantage, such as ability to survive in a markedly acid- or bile-rich environment, leads to dominant and widespread clones. Once initiated, the promotion and propagation of metaplastic clones is dependent on the surrounding microenvironment, particularly the presence of a chronic inflammatory cell infiltrate, characterised by T lymphocytes, and cytokines such as interleukin-1, tumour necrosis factor-α and transforming growth factor-β. These lead to an increase in cyclo-oxygenase-2, c-myc and cyclin D1, which increase proliferation and decrease apoptosis, and a reduction in E-cadherin, with resultant loss of cell adhesion and localisation of β-catenin to the nucleus.28 These molecular changes underlie the progression of Barrett’s oesophagus to cancer via the metaplasia–dysplasia–adenocarcinoma sequence (see Fig. 15.1). Figure 15.1 The metaplasia–dysplasia–adenocarcinoma sequence. There are histological stages of progression (shaded rectangles representing the clonal expansion of competing stem cells). In addition there are structural genetic changes in the form of mutations (vertical arrows) and environmental changes (white rectangles) driving cell cycle and cell adhesion biological sequelae. APC, adenomatous polyposis coli gene; Cdx, CauDal protein gene; LOH, loss of heterozygosity; RERs, random errors of replication; TGF-β, transforming growth factor-β. Adapted from Jankowski J, Harrison RF, Perry I et al. Barrett’s metaplasia. Lancet 2000; 356:2079–85. With permission from Elsevier. Although traditionally thought of as an acquired condition, genetic factors may play a part in a small proportion of patients with Barrett’s metaplasia, as family and twin studies suggest a subgroup of individuals with a strong familial tendency to Barrett’s oesophagus.29,30 A family in the UK has been identified with a male index case with oesophageal adenocarcinoma, three brothers with Barrett’s-associated cancer or HGD, and six children with Barrett’s oesophagus.31 Linkage studies are being undertaken in order to further our understanding of this genetic inheritance. However, there are data to suggest Barrett’s is a polygenic disease with multiple contributing genes acting together. Barrett’s oesophagus is accepted as a significant risk factor for adenocarcinoma of the oesophagus, although the risk of progression to adenocarcinoma and the risk of disease-specific mortality is low. A large number of studies have estimated the risk of adenocarcinoma arising from Barrett’s oesophagus, with very variable results.32–42 Former studies included small numbers of patients and were likely subject to publication bias, with results only being published if they showed a high incidence of cancer, leading to an overestimate of risk.43 Recently, two large population-based cohort studies have reported much lower annual rates of progression to adenocarcinoma (0.12–0.13% per year) than in former series (Table 15.2). It should be noted that these figures exclude carcinomas of the gastric cardia and also do not reflect progression to HGD. In addition, the two studies used different approaches to select patients with Barrett’s oesophagus. The study by Hvid-Jensen et al.33 identified patients with intestinal metaplasia (IM) from the Danish National Pathology Registry without corroboration with endoscopic findings. Therefore, potentially, patients may have been included who had a diagnosis of cardiac IM rather than true Barrett’s oesophagus, producing an incorrect denominator and leading to a slight underestimate of the risk of disease progression. Bhat et al.32 included patients with columnar-lined oesophagus (CLO) at endoscopy (although the validated Prague system was not used), which was corroborated histologically, and demonstrated an increased risk of progression in patients who had IM confirmed histologically at index endoscopy, compared to those with CLO without IM. This finding is in keeping with previous studies demonstrating a higher risk of disease progression in patients with confirmed IM.1,44,45 Previous studies have, however, suggested a significant geographical variation in the incidence of carcinoma arising in Barrett’s oesophagus in Western countries, with incidence rates in the UK almost double those in the USA.46 It is also worth noting that the population demographic in Denmark differs somewhat from the USA and UK, where rates of obesity are significantly higher and where a greater proportion of men, who are at higher risk of malignant progression, develop Barrett’s oesophagus. A recent meta-analysis reported a pooled estimate of the annual risk of cancer progression in non-dysplastic Barrett’s of 0.39% per year. Importantly, only eight of 47 studies that met all three quality criteria were included in this analysis; inclusion of the remaining studies significantly increased this figure.35 The risk in the UK following a meta-analysis is indicated as closer to 1%, higher than is reported in the USA.46 When considering the natural history of dysplasia in Barrett’s oesophagus we must remember that in addition to potential problems with length of follow-up and sampling error at endoscopy, there is considerable inter- and intra-observer variation among experienced pathologists in the histological diagnosis of dysplasia. While pathologists can demonstrate acceptable levels of agreement in distinguishing HGD combined with carcinoma from no dysplasia combined with indefinite and low-grade dysplasia (kappa values of 0.8), there are much poorer levels of agreement in distinguishing between the four groups: indefinite for dysplasia, LGD, HGD and carcinoma (intra-observer kappa values of 0.43–0.64).48 Pathologists find it particularly difficult to separate inflammation in Barrett’s oesophagus from LGD. In this situation pathologists should be encouraged to make use of the indefinite for dysplasia category: such a diagnosis does not mean that the pathologist is uncertain, but rather that it is not possible, with confidence, to exclude LGD in inflamed material. The diagnosis of HGD has serious implications for patient management and the diagnosis should be confirmed by two expert pathologists. The natural history of LGD is not fully understood and reported rates of regression/progression vary considerably, reflecting the diagnostic difficulties discussed above. Sharma et al.50 followed 156 patients for a mean of 4.1 years and reported progression to HGD or cancer in 13%, regression in 66% and stable LGD in 21%. A more recent prospective cohort study of 713 Barrett’s patients, including 111 with LGD, reported that compared to non- dysplastic disease, LGD was a significant risk factor for progression to HGD or adenocarcinoma (relative risk (RR) 9.7; 95% confidence interval (CI) 4.4–21.5).51 Similarly, in their large population- based study, Hvid-Jensen et al.33 reported that the relative risk of oesophageal cancer among those who had LGD at baseline, as compared to those without LGD at baseline, was 4.8 (95% CI 2.6–8.8). The annual risk of progression to HGD or cancer was found to be 1.27% for those with LGD at baseline. Bhat et al.32 reported a hazard ratio of 5.67% (95% CI 3.77–8.33) for patients with LGD compared to no dysplasia. However, a recent study by Wani et al.,52 which followed up 210 patients with Barrett’s oesophagus with or without LGD for a mean of 6.2 years, found no associations of presence of prevalent, incident or persistent LGD, or the extent of LGD, with progression rates. Bergman and colleagues recently demonstrated that LGD is over-diagnosed by non-specialist pathologists and argued that its true significance might have been underestimated by many reported series.53 In their study, 1198 patients underwent Barrett’s surveillance at six non-specialist hospitals, identifying 147 (12.5%) patients with LGD. However, only eight (0.7%) patients were deemed to have LGD following histological review by two external expert gastrointestinal pathologists. The majority of diagnoses were reclassified as non- dysplastic Barrett’s oesophagus. During a mean follow-up period of 51 months, 42% of patients with LGD diagnosed by consensus expert pathologists demonstrated progression to either carcinoma or HGD, and 2.2% regressed to non-dysplastic Barrett’s oesophagus.53 Studies reporting the natural history of HGD have also reported widely differing results. Reid et al.54 followed 76 patients for 5 years and reported that 59% developed adenocarcinoma. In a study of 100 patients with HGD, 66 of whom underwent surveillance, 3 of 24 patients (13%) with focal HGD and 17 of 42 patients (40%) with diffuse HGD developed carcinoma after a mean follow-up of 41 and 23 months, respectively.55 An important question to consider is what proportion of patients with a diagnosis of HGD who undergo oesophagectomy have an occult cancer detected in the resected specimen? Table 15.3 shows reported rates in the literature of 0–73%: overall the rate appears to be approximately 40%.56–72 Patients with visible, nodular HGD appear at greatest risk of harbouring coexisting cancer.73,74 This emphasises the fact that patients with HGD may be harbouring an undetected cancer and confirms the need for complete staging in these patients. Table 15.3 Studies reporting the incidence of adenocarcinoma in resected specimens following oesophagectomy for high-grade dysplasia IMC, intramucosal cancer; inv. ca., invasive cancer (denotes invasion into submucosa or beyond). Given that endotherapy is becoming a recognised treatment option for focal intramucosal cancers (T1a), a more pertinent question to ask might be: what is the prevalence of submucosal invasive cancer at oesophagectomy for HGD? The majority of studies in Table 15.3 make no attempt to separate intramucosal cancer (IMC) from more advanced lesions; however, some more recent reports suggest that rates of invasive cancer (submucosa or beyond) are considerably lower than 40%. Wang et al.71 retrospectively assessed 60 patients (41 with preoperative HGD and 19 with preoperative IMC) who underwent oesophagectomy. The overall rate of submucosal cancer was 6.7%, with a rate of 5% in patients with preoperative HGD and 11% in patients with preoperative IMC. Only one patient (1.7%) had nodal metastasis. Another recent study found the rate of invasive adenocarcinoma (excluding IMC) in association with Barrett’s HGD to be 11.7% (8/68), with 5.9% having occult cancer.75 Although some HGD may be stable or even regress, between 15% and 59% will progress to adenocarcinoma over 5 years. However, if detailed biopsy mapping endoscopies showed no previous HGD (prevalent HGD), then the detection of new HGD (incident HGD) is associated with a risk of subsequent progression to cancer of only between 3% and 5% per year.73,76 This area is being actively discussed in the Barrett’s Dysplasia and Cancer Taskforce (BAD CAT) group. The length of Barrett’s segment has been shown to be a significant risk factor for progression to cancer, a doubling of length increasing the risk 1.7-fold.77 The extent of HGD and/or LGD also appears to be a risk factor for progression to adenocarcinoma.55,78 Importantly, in a prospective longitudinal cohort study, individuals with Barrett’s oesophagus who were regularly taking aspirin or other NSAIDs were found to have a significantly lower 5-year cumulative incidence of adenocarcinoma compared with individuals not taking NSAIDs (6.6% and 14.3%, respectively), suggesting that this may be an effective chemotherapeutic intervention.79 An ongoing phase III multicentre randomised controlled trial (RCT), the AspECT trial (Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia), designed to test this hypothesis is due to report in 2016. The primary aim of this study is to determine whether acid suppression with proton-pump inhibition (high dose vs. low dose) with or without aspirin can reduce mortality or the conversion from Barrett’s metaplasia to HGD or adenocarcinoma. Both high- and low-dose acid suppression are being investigated as there remains doubt about the optimal dose of proton-pump inhibitor (PPI) to use, especially given the fact that Barrett’s mucosa is relatively insensitive, thus rendering symptoms unreliable. There is an argument that incomplete acid suppression might increase the risk of cancer by exposing the mucosa to short pulses of acid, thus stimulating the proliferation of abnormal cells. In contrast, there is some epidemiological evidence that high-dose proton-pump inhibition might increase the risk of cancer as bile acid might become cytotoxic at neutral pH. In addition, there have been fears that PPI-induced hypergastrinaemia could stimulate hyperproliferation of Barrett’s epithelium.80,81 Although this risk is yet to be evaluated in vivo, it appears more likely that gastrin induces epithelial restitution in Barrett’s oesophagus, without stimulation of clonal expansion or disease progression.82 It is accepted that GORD is a significant risk factor for the development of adenocarcinoma, with a well-known Swedish case–control study demonstrating a 44-fold increased relative risk in individuals with frequent heartburn of greater than 20 years’ duration.17 This has led to the suggestion that screening individuals with chronic reflux symptoms to detect Barrett’s oesophagus and cancer may be of benefit. However, it is important to appreciate two flaws in this concept: firstly, approximately 40% of individuals with cancer in the series mentioned above denied frequent heartburn; secondly, a significant proportion of individuals with Barrett’s oesophagus are asymptomatic. In addition, Barrett’s patients experience less heartburn and use PPIs less frequently compared with controls.2,83,84 The endoscopic screening of individuals with chronic reflux symptoms to detect either Barrett’s or cancer is not currently recommended in the UK or USA.1,2 This is because of the low absolute risk of developing adenocarcinoma in individuals with chronic reflux, combined with the knowledge that most individuals with Barrett’s oesophagus die from causes other than oesophageal cancer. There are also concerns about the cost-effectiveness and invasiveness of endoscopy as a screening tool. Several attempts have been made to develop a scoring system using patient demographics and symptoms to predict the presence of Barrett’s oesophagus for screening purposes.85,86 However, interest in these risk prediction strategies has declined due to inability to generate sufficient sensitivity and specificity. Mutations in the p53 tumour suppressor gene are widely found in dysplastic Barrett’s oesophagus and oesophageal cancer. Younes et al.87 found p53 mutation in 9% of Barrett’s patients with LGD, 55% of patients with HGD and 87% of patients with carcinoma: no patients without dysplasia had a p53 mutation. Importantly, in a further study, 56% of patients with LGD and p53 mutation progressed to HGD or carcinoma, whereas no patient with LGD without p53 mutation progressed.88 Similarly, Reid et al.89 demonstrated that loss of heterozygosity of gene 17 (p53) was found in 6% of patients without dysplasia, 20% of patients with LGD and 57% of patients with HGD. Patients with loss of heterozygosity had a 16-fold increased risk of cancer after 3 years. These results have led to the suggestion that the subgroup of patients with low-grade or indefinite dysplasia and p53 mutation should be subjected to more rigorous surveillance protocols. However, it is important to remember that not all oesophageal adenocarcinomas express p53, and patients without expression can progress to cancer. Other markers that have been identified as conferring a high risk of progression are p16 mutations,90 cyclin D1 overexpression,91 flow cytometry abnormalities such as aneuploidy and increase in the G2/tetraploidy fraction of DNA content,92 and reduced expression of E-cadherin, with resultant loss of cell adhesion and localisation of β-catenin to the nucleus.93 Several clinical trials are under way, including the Chemoprevention of Premalignant Intestinal Neoplasia (ChOPIN) trial and the Barrett’s Oesophagus Screening Trial (BEST2) trial, which aim to explore non-invasive methods of screening for malignant progression. ChOPIN aims to detect a panel of predictive serum biomarkers, whereas BEST2 is a case–control trial investigating the potential of a non-endoscopic immunocytological device (Cytosponge).94,95 This trial requires patients to swallow a small capsule that dissolves into a 3-cm sponge in the stomach and is then withdrawn through the oesophagus. Oesophageal cells are assessed for a range of predictive biomarkers, including TFF3 positivity (the principal end-point) as well as ploidy, Mcm2, cyclin A, TP53 and methylation. Cost data and the impact of screening on psychosocial well-being are also being evaluated. It is hoped that non-invasive screening tests such as these could enable safe, accurate and cost-effective population-based screening in the future.
Barrett’s oesophagus
Definition
Epidemiology
Endoscopic assessment
Imaging modality
Concept
Reference
White light endoscopy
High-resolution magnification endoscopy (HRME)
Greater magnification and resolution than normal endoscopy allowing more detailed visualisation of the mucosa
May et al. (2004)137
Chromoendoscopy
Topical application of dyes improves visualisation of mucosal surfaces. Examples: methylene blue – absorbed with different patterns into different types of mucosa; indigo carmine – accumulates in mucosal fissures accentuating surface topography
Canto et al. (2006)138
Optical endoscopy
Autofluorescence imaging (AFI)
Short-wavelength light causes excitation of endogenous biological tissues with subsequent release of longer wavelength fluorescent light
Kara et al. (2005)139
Narrow-band imaging (NBI)
Narrow-bandwidth green and blue light (with exclusion of red light) only superficially penetrates mucosa, improving visualisation of mucosal microvasculature and surface morphology
Curvers et al. (2008)25
Confocal microscopy (CM)
Real-time magnification of the mucosa up to 1000-fold enables visualisation of cellular structures
Dunbar and Canto (2010)22
Elastic scattering spectroscopy (ESS)
Elastic scattering of white light generates real-time morphological information about the size and shape of the cell nuclei and the degree of cellular crowding in the mucosa and submucosa
Qiu et al. (2010)23
Trimodal imaging
Incorporates HRME, AFI and NBI in a single endoscope with ability to switch between modalities during procedure
Curvers et al. (2010, 2011)140,141
Molecular imaging
Fluorescently tagged molecular probes bind selectively to metaplastic or dysplastic cells
Bird-Lieberman et al. (2012)142
Pathophysiology of Barrett’s oesophagus and progression to adenocarcinoma
Risk of cancer and mortality in Barrett’s oesophagus
Natural history of dysplasia in Barrett’s oesophagus
Low-grade dysplasia
High-grade dysplasia
Risk factors for progression to cancer
Screening for Barrett’s oesophagus and adenocarcinoma using molecular markers
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Abdominal Key
Fastest Abdominal Insight Engine