Bacterial Infections of the Genitourinary Tract: Introduction
Urinary tract infection (UTI) is a term that is applied to a variety of clinical conditions ranging from the asymptomatic presence of bacteria in the urine to severe infection of the kidney with resultant sepsis. UTI is one of the more common medical problems. It is estimated that 150 million patients are diagnosed with a UTI yearly, resulting in at least $6 billion in health care expenditures (Stamm and Norrby, 2001). UTIs are at times difficult to diagnose; some cases respond to a short course of a specific antibiotic, while others require a longer course of a broad-spectrum antibiotic. Accurate diagnosis and treatment of a UTI is essential to limit its associated morbidity and mortality and avoid prolonged or unnecessary use of antibiotics. Advances in our understanding of the pathogenesis of UTI, the development of new diagnostic tests, and the introduction of new antimicrobial agents have allowed physicians to appropriately tailor specific treatment for each patient.
Epidemiology
The epidemiology of UTI grouped by age and sex is shown in Table 14–1. In newborns up to 1 year of age, bacteriuria is present in 2.7% of boys and 0.7% in girls (Wettergren et al, 1985). The incidence of UTI in uncircumcised males is higher than in circumcised males (1.12% compared with 0.11%) during the first 6 months of life (Wiswell and Roscelli, 1986). In children between 1 and 5 years of age, the incidence of bacteriuria in girls increases to 4.5%, while it decreases in boys to 0.5% (Randolph and Greenfield, 1964). Most UTIs in children younger than 5 years are associated with congenital abnormalities of the urinary tract, such as vesicoureteral reflux or obstruction. The incidence of bacteriuria remains relatively constant in children 6–15 years of age. However, the UTIs in these children are more likely to be associated with functional abnormalities of the urinary tract, such as dysfunctional voiding. During adolescence, the incidence of UTI significantly increases (to 20%) in young women, while remaining constant in young men (Sanford, 1975).
Incidence (%) | |||
|---|---|---|---|
Age (y) | Female | Male | Risk factors |
<1 | 0.7 | 2.7 | Foreskin, anatomic GU abnormalities |
1–5 | 4.5 | 0.5 | Anatomic GU abnormalities |
6–15 | 4.5 | 0.5 | Functional GU abnormalities |
16–35 | 20 | 0.5 | Sexual intercourse, diaphragm use |
36–65 | 35 | 20 | Surgery, prostate obstruction, catheterization |
>65 | 40 | 35 | Incontinence, catheterization, prostate obstruction |
Approximately 7 million cases of acute cystitis are diagnosed yearly in young women (Schappert, 1999); this likely is an underestimate of the true incidence of UTI because at least 50% of all UTIs do not come to medical attention. The major risk factors for women 16–35 years of age are related to sexual intercourse and diaphragm use. Later in life, the incidence of UTI increases significantly for both males and females. For women between 36 and 65 years of age, gynecologic surgery and bladder prolapse appear to be important risk factors. In men of the same age group, prostatic hypertrophy/obstruction, catheterization, and surgery are relevant risk factors. For patients older than 65 years, the incidence of UTI continues to increase in both sexes. Incontinence and chronic use of urinary catheters are important risk factors in these patients. In those younger than 1 year and those older than 65 years, the morbidity and mortality of UTI are the greatest (Shortliffe and McCue, 2002).
Based on data from the Urologic Diseases in North America Project, the overall lifetime prevalence of UTI was estimated to be 14,000 per 100,000 men (Griebling, 2005a) and 53,000 per 100,000 women (Griebling, 2005b). Overall medical expenditures for the treatment of UTIs in the United States were estimated to be $1 billion for men (Griebling, 2005a) and $2.5 billion for women (Griebling, 2005b). The increased cost in treatment of UTIs for women is primarily due to an increase in the trend toward using fluoroquinolones as a first-line therapy for UTI. UTIs occurred in 2.4–2.8% of children. In this patient population, UTIs resulted in more than 1.1 million physician visits annually, accounting for 0.7% of the doctor visits (Freedman, 2005).
Pathogenesis
Understanding of the mode of bacterial entry, host susceptibility factors, and bacterial pathogenic factors is essential to tailoring appropriate treatment for the diverse clinical manifestations of UTI. There are four possible modes of bacterial entry into the genitourinary tract. It is generally accepted that periurethral bacteria ascending into the urinary tract causes most UTI. Most cases of pyelonephritis are caused by the ascent of bacteria from the bladder, through the ureter and into the renal parenchyma. Consequently, the short nature of the female urethra combined with its close proximity to the vaginal vestibule and rectum likely predisposes women to more frequent UTIs than men (Nicolle et al, 1982).
Other modes of bacterial entry are uncommon causes of UTI. Hematogenous spread can occur in immunocompromised patients and in neonates. Staphylococcus aureus, Candida species, and Mycobacterium tuberculosis are common pathogens that travel through the blood to infect the urinary tract. Lymphatogenous spread through the rectal, colonic, and periuterine lymphatics has been postulated as a cause for UTI; however, currently, there is little scientific support to suggest that dissemination of bacteria through lymphatic channels plays a role in the pathogenesis of UTI. Direct extension of bacteria from adjacent organs into the urinary tract can occur in patients with intraperitoneal abscesses or vesicointestinal or vesicovaginal fistulas. Relapsing infection from an inadequately treated focus in the prostate or kidney may seed other parts of the urinary tracts.
Host factors have an essential role in the pathogenesis of UTI. Unobstructed urinary flow with the subsequent washout of ascending bacteria is essential in preventing UTI. In addition, the urine itself has specific characteristics (its osmolality, urea concentration, organic acid concentration, and pH) that inhibit bacterial growth and colonization (Sobel, 1997). It also contains factors that inhibit bacterial adherence, such as Tamm–Horsfall glycoprotein (THG; Duncan, 1988; Pak et al, 2001; Wagenlehner et al, 2005). Is has been observed that the severity of the bacteriuria and the degree of inflammatory changes in the urinary tract were much greater in THG-deficit mice, suggesting that THG helps eliminate bacterial infection from the urinary tract and acts as a general host-defense factor against UTI (Raffi et al, 2005). Urinary retention, stasis, or reflux of urine into the upper urinary tract can promote bacterial growth and subsequent infection. Consequently, any anatomic or functional abnormalities of the urinary tract that impede urinary flow can increase the host’s susceptibility to UTI. These abnormalities include obstructive conditions at any level of the urinary tract, neurologic diseases affecting the function of the lower urinary tract, diabetes, and pregnancy. Similarly, the presence of foreign bodies (such as stones, catheters, and stents) allows the bacteria to hide from these host defenses.
The epithelium lining the urinary tract not only provides a physical barrier to infection but also has the capacity to recognize bacteria in order to innate host defenses. The urothelial cells express toll-like receptors (TLRs) that upon engagement by specific bacterial components lead to production of inflammatory mediators (Chowdhury et al, 2004). In response to the presence of bacteria, cells lining the urinary tract secrete chemoattractants such as interleukin-8 to recruit neutrophils to the area and limit tissue invasion (Frendeus et al, 2001). Specific serum and urinary antibodies are produced by the kidney to enhance bacterial opsonization and phagocytosis and to inhibit bacterial adherence. The protective role of both cellular and humoral-mediated immunity in preventing UTIs remains unclear; deficiency in B-cell or T-cell function has not been associated with the increased frequency of UTI or altered the course of the infection (Schaeffer, 2001; Svanborg Eden et al, 1988). However, it should be noted that the same host-defense mechanisms that help to prevent/limit the infection (such as the inflammatory responses) can lead to cell and tissue damage. In the kidneys, cell damage and subsequent development of scarring may lead to pathological conditions such as hypertension, preeclampsia during pregnancy, and renal dysfunction and failure (Jahnukainen et al, 2005).
Many studies have demonstrated that there is selectivity in bacterial adherence to cells lining the urinary tract, and the degree of adherence correlates with colonization and infection. Women with recurrent UTIs have higher adherence of bacteria to their mucosal cells in vitro compared with women who never had an infection (Navas et al, 1994). The increased adherence may be due to having more binding sites for bacterial adhesins on their mucosal cells. Alternatively, these patients may not secrete soluble compounds, which normally compete for the same receptors that bind bacterial adhesins. Blood group antigens may constitute one group of these soluble compounds that inhibit bacterial adherence (Lomberg et al, 1986). These findings would suggest a genetic predisposition for UTI.
Other important host factors include the normal flora of the periurethral area or the prostate and the presence of vesicoureteral reflux. In women, the normal flora of the periurethral area composed of organisms such as lactobacillus provides a defense against the colonization of uropathogenic bacteria (Osset et al, 2001). Alterations in the periurethral environment (such as changes in the pH or estrogen levels or the use of antibiotics) can damage the periurethral flora, allowing uropathogens to colonize and subsequently to infect the urinary tract (Schaeffer et al, 1999). In men, the prostate secretes fluid containing zinc, which has potent antimicrobial activity (Fair et al, 1976). Finally, in children, the presence of vesicoureteral reflux does not increase their susceptibility to UTI but does allow bacteria to be inoculated into the upper tract and the infection to progress.
Aging is associated with an increased susceptibility to UTI, in part because of the increased incidence of obstructive uropathy in men (Matsumoto, 2001; Nicolle, 2002) and alteration in the vaginal and periurethral flora from menopause in women (Foxman et al, 2001). Other causes include soiling of the perineum from fecal incontinence, neuromuscular diseases, increased instrumentation, and bladder catheterization (Ronald, 2002).
Not all bacteria are capable of adhering to and infecting the urinary tract. Of the many strains of Escherichia coli, the uropathogens belong to a limited number of O, K, and H serogroups. They have increased adherence properties to uroepithelial cells (Blanco et al, 1996; Hovanec and Gorzynski, 1980; Orskov et al, 1982), resistance to the bactericidal activity of human serum (Bjorksten and Kaijser, 1978), production of hemolysin (Hughes et al, 1983; Koronakis and Hughes, 1996), and the increased expression of K capsular antigen (Whitfield and Roberts, 1999). The ability of E. coli to adhere to epithelial cells is mediated by ligands located on the tips of the bacterial fimbriae (pili). The ligands bind to glycolipids or glycoprotein receptors on the surface membrane of uroepithelial cells. The pili are classified by their ability to cause hemagglutination and the type of sugar that can block this process. P pili, which can agglutinate human blood, bind to glycolipid receptors on uroepithelial cells, erythrocytes (P blood group antigens), and renal tubular cells (Svenson et al, 1983). Type 1 pili, which can agglutinate guinea pig blood, bind to mannoside residues on uroepithelial cells (Ofek et al, 2000). P pili are observed in >90% of the E. coli strains causing pyelonephritis but <20% of the strains causing lower UTIs (Kallenius et al, 1981; Roberts et al, 1997a). In contrast, type 1 pili may help bacteria to adhere to bladder mucosa (Connell et al, 1996; Martinez et al, 2000). Most uropathogenic E. coli have both types of pili. Once attachment to the uroepithelial cells occurs, other bacterial pathogenic factors become important. Most uropathogenic E. coli strains produce hemolysin, which initiates tissue invasion and makes iron available for the infecting pathogens (Hughes et al, 1983; Koronakis and Hughes, 1996). The presence of K antigen on the invading bacteria protects them from phagocytosis by neutrophils (Bortolussi et al, 1979; Evans et al, 1981). These factors allow the infecting pathogens to escape the various host defenses (Svanborg et al, 1996).
It has been observed that many bacteria such as E. coli have the ability to invade into the host cells, acting as opportunistic intracellular pathogens (Bower et al, 2005). Cytotoxic necrotizing factor, Afa/Dr adhesions, and type 1 pili have been shown to promote invasion into the host cells. Binding of FimH adhesin at the distal tip of type 1 pili to the host membrane leads to the recruitment of focal adhesin kinase, phosphoinositide-3-kinases, α-actinin, and vinculin, which results in localized actin rearrangements and engulfment of the bound bacterium by zippering of the membrane around the bacteria (reviewed by Anderson et al, 2004). The intracellular bacteria mature into biofilms, creating pod-like bulges on the urothelial surface. The pods contain bacteria encased in a polysaccharide-rich matrix surrounded by a protective shell of uroplakin. The ability of the uropathogenic bacteria to transiently invade, survive, and multiply within the host cells and to create biofilms on genitourinary tract tissues may provide a mechanism for the persistence and recurrence of UTIs.
Causative Pathogens
Most UTIs are caused by a single bacterial species. At least 80% of the uncomplicated cystitis and pyelonephritis are due to E. coli, with most of pathogenic strains belonging to the O serogroups (Orskov et al, 1982). Other less common uropathogens include Klebsiella, Proteus, and Enterobacter spp. and enterococci. In hospital-acquired UTIs, a wider variety of causative organisms is found, including Pseudomonas and Staphylococcus spp. (Wagenlehner and Naber, 2000); UTIs caused by S. aureus often result from hematogenous dissemination. Group B beta-hemolytic streptococci can cause UTIs in pregnant women (Wood and Dillon, 1981). Staphylococcus saprophyticus, once often thought of as urinary contaminants, can cause uncomplicated UTIs in young women (Hovelius and Mardh, 1984). In children, the causative bacterial spectrum is slightly different from that of adults, with Klebsiella and Enterobacter spp. being more common causes of UTI (Jeena et al, 1996; Ronald, 2002; Schlager, 2001). Anaerobic bacteria, lactobacilli, corynebacteria, streptococci (not including enterococci), and Staphylococcus epidermidis are found in normal periurethral flora. They do not commonly cause UTIs in healthy individuals and are considered common urinary contaminants.
Diagnosis
The diagnosis of UTI is sometimes difficult to establish and relies on urinalysis and urine culture. Occasionally, localization studies may be required to identify the source of the infection. Most often, the urine is often obtained from a voided specimen. In children who are not toilet trained, a urine collection device, such as a bag, is placed over the genitalia, and the urine is cultured from the bagged specimen. These two methods of urine collection are easy to obtain, but potential contamination from the vagina and perirectal area may occur. There is a high false-positive rate, especially from bagged specimens (Al-Orifi et al, 2000). Suprapubic aspiration avoids potential contamination; however, due to its invasiveness, it is rarely used except in children and selected patients. Urine obtained from a urinary catheter is less invasive than a suprapubic aspiration and is less likely to be contaminated than that obtained from a voided specimen. If a patient has an indwelling catheter, a urine specimen should be obtained from the collection port on the catheter.
The urine can be immediately evaluated for leukocyte esterase, a compound produced by the breakdown of white blood cells (WBCs) in the urine. Urinary nitrite is produced by reduction of dietary nitrates by many gram-negative bacteria. Esterase and nitrite can be detected by a urine dipstick and are more reliable when the bacterial count is >100,000 colony-forming units (CFUs) per milliliter. Microscopic examination of the urine for WBCs and bacteria is performed after centrifugation. When bacteria counts are >100,000 CFU/mL, bacteria can be detected microscopically (Jenkins et al, 1986). More than three WBCs per high-power field suggest a possible infection. The sensitivity and specificity of these tests are shown in Table 14–2 (Williams et al, 2010). The urinary nitrite test is highly specific but not sensitive, whereas the other three tests have a sensitivity and specificity approximately 80%. A combination of these tests may help to identify those patients in whom urine culture will be positive. Conversely, when esterase, nitrite, blood, and protein are absent in a urine sample, <2% of the urine samples will be positive by culture, providing a >98% negative predictive value and a sensitivity of 98% (Patel et al, 2005). A recent systematic review of the literature indicates that urinalysis is more effective in the diagnosis of UTI as determined by urine culture in children older than 2 years than for younger children (Mori et al, 2010).
Tests | Sensitivity (%) | Specificity (%) |
|---|---|---|
Esterase (E) | 79 (73–84) | 87 (80–92) |
Nitrite (N) | 49 (41–57) | 98 (96–99) |
E or N | 88 (82–91) | 79 (69–87) |
E + N | 45 (30–61) | 98 (96–99) |
WBC | 74 (67–80) | 86 (82–90) |
Bacteria | 88 (75–94) | 92 (83–96) |
The gold standard for identification of UTI is the quantitative culture of urine for specific bacteria. The urine should be collected in a sterile container and cultured immediately after collection. When this is not possible, the urine can be stored in the refrigerator for up to 24 hours. The sample is then diluted and spread on culture plates. Each bacterium will form a single colony on the plates. The number of colonies is counted and adjusted per milliliter of urine (CFU/mL). Defining the CFU/mL that represents clinically significant infection can be difficult. It is dependent on the method of collection, the sex of the patient, and the type of bacteria isolated (Table 14–3). Traditionally, >100,000 CFU/mL is used to exclude contamination. However, studies have clearly demonstrated that clinically significant UTI can occur with <100,000 CFU/mL bacteria in the urine (Stamm et al, 1982).
Collection | CFU | Probability of infection (%) |
|---|---|---|
Suprapubic | Gram negative any Gram positive >1000 | >99 |
Catheterization | >105 | 95 |
104–5 | Likely | |
103–4 | Repeat | |
<103 | Unlikely | |
Clean catch | ||
Male | >104 | Likely |
Female | 3 specimens: >105 | 95 |
2 specimens: >105 | 90 | |
1 specimen: >105 | 80 | |
5 × 104–105 | Repeat | |
1–5 × 104 symptomatic | Repeat | |
1–5 × 104 nonsymptomatic | Unlikely | |
<104 | Unlikely |
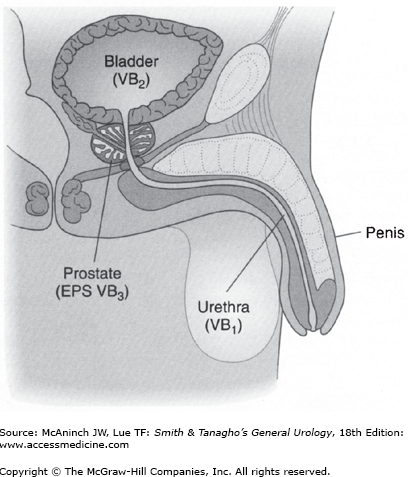
Occasionally, it is necessary to localize the site of infection. For upper urinary tract localization (Lorentz, 1979), the bladder is irrigated with sterile water and a ureteral catheter is placed into each ureter. A specimen is collected from the renal pelvis. Culture of this specimen will indicate whether infection in the upper urinary tract is present. In men, infection in the lower urinary tract can be differentiated (Figure 14–1) (Meares and Stamey, 1968). A specimen is collected at the beginning of the void and represents possible infection in the urethra. A midstream specimen is next collected and represents possible infection in the bladder. The prostate is then massaged and the patient is asked to void again. This specimen represents possible infection of the prostate.
Antibiotics
Treatment with antimicrobial agents has minimized the morbidity and mortality associated with UTIs. The goal in treatment is to eradicate the infection by selecting the appropriate antibiotics that would target specific bacterial susceptibility. However, choosing the appropriate antimicrobial agents is often difficult. Many antibiotics are available, and the lowest effective dose and length of therapy are not well defined. Many conventions for the treatment of UTI are arbitrary. The general principles for selecting the appropriate antibiotics include consideration of the infecting pathogen (antibiotic susceptibility, single-organism vs poly-organism infection, pathogen vs normal flora, community vs hospital-acquired infection); the patient (allergies, underlying diseases, age, previous antibiotic therapy, other medications currently taken, outpatient vs inpatient status, pregnancy); and the site of infection (kidney vs bladder vs prostate). Because most antibiotics are cleared from the body by the liver or the kidney, certain antimicrobial agents need to be adjusted in the presence of liver or renal diseases (Table 14–4). Table 14–5 lists the common uropathogens and the recommended oral and intravenous antimicrobial agents for treatment. Table 14–6 lists the common sites of UTI, the recommended treatment, and the duration of therapy. In patients with recurrent UTIs or those who are at risk for UTI (such as children with vesicoureteral reflux), prophylactic antibiotics may be used. Table 14–7 lists common prophylactic regimens.
Renal diseases (Cr clearance <30 mL/min) |
Aminoglycosides |
Beta-lactams |
Cefoxitin, ceftizoxime |
Cefonicid, ceftazidime |
Cefuroxime, cefepime |
Cefpirome, moxalactam |
Carbenicillin, ticarcillin, ticarcillin–clavulanate |
Vancomycin |
Tetracyclines (except doxycycline) |
Sulfonamides |
Hepatic diseases (with elevated bilirubin) |
Chloramphenicol |
Tetracyclines |
Clindamycin, rifampin, pefloxacin |
Renal–hepatic diseases |
Ceftriaxone |
Cefoperazone |
Carbenicillin |
Ticarcillin |
Azlocillin |
Mezlocillin |
Piperacillin |
Bacteria | Oral therapy | Parenteral therapy |
|---|---|---|
Gram-positive cocci | ||
Staphylococcus aureus | Nafcillin, nitrofurantoin, ciprofloxacin | Nafcillin, vancomycin |
Staphylococcus epidermidis | Ampicillin, nitrofurantoin, ciprofloxacin | Ampicillin, penicillin G |
Staphylococcus saprophyticus | Ampicillin, nitrofurantoin, ciprofloxacin | Ampicillin, penicillin G |
Streptococcus, group D S. faecalis (enterococci) S. bovis | Ampicillin, nitrofurantoin Penicillin G, ampicillin | Ampicillin plus gentamicin Ampicillin, vancomycin |
Streptococcus, group B | Ampicillin, cephalosporin | Ampicillin, cephalosporin |
Gram-negative cocci | ||
Neisseria gonorrhoeae | Ciprofloxacin plus doxycycline | Ceftriaxone |
Gram-negative rods | ||
Escherichia coli | TMP-SMX, ciprofloxacin, nitrofurantoin | Gentamicin |
Enterobacter spp. | TMP-SMX, ciprofloxacin, nitrofurantoin | Gentamicin plus piperacillin |
Gardnerella vaginalis | Metronidazole, ampicillin | Metronidazole |
Klebsiella spp. | TMP-SMX, ciprofloxacin | Gentamicin plus cephalosporin |
Proteus spp. | Ampicillin, TMP-SMX, ciprofloxacin | Ampicillin, gentamicin |
Pseudomonas aeruginosa | Carbenicillin, tetracycline, ciprofloxacin | Gentamicin plus piperacillin |
Serratia spp. | TMP-SMX, carbenicillin | TMP-SMX, amikacin |
Other pathogens | ||
Chlamydiae | Tetracycline, erythromycin | Tetracycline, erythromycin |
Mycoplasmas, ureaplasmas | Tetracycline, erythromycin | Tetracycline, erythromycin |
Obligate anaerobes | Metronidazole, clindamycin | Metronidazole, clindamycin |
Diagnosis | Pathogen | Choice of antibiotics | Duration of therapy |
|---|---|---|---|
Cystitis | E. coli Klebsiella Proteus | 1st: TMP-SMX 2nd: Fluoroquinolone | 1–3 days |
Pyelonephritis | E. coli Proteus Klebsiella Enterobacteria | 1st: Gluoroquinolone 2nd: 2nd generation cephalosporin 3rd: Aminopenicillin/BLI | 7–10 days |
Complicated UTI | E. coli Enterococci Pseudomonas Staphylococci | 1st: Fluoroquinolone 2nd: Aminopenicillin/BLI 3rd: 3rd generation cephalosporin Aminoglycosides | 3–5 days after afebrile |
Prostatitis | E. coli Enterobacteria Pseudomonas Enterococci | 1st: Fluoroquinolone 2nd: 2nd generation cephalosporin 3rd: 3rd generation cephalosporin | Acute: 2 weeks Chronic: 4–6 weeks |
Epididymitis | E. coli Enterobacteria Enterococci Chlmaydia Ureaplasma | 1st: Fluoroquinolone 2nd: 2nd generation cephalosporin 1st: Doxycycline 2nd: Macrolide | 2 weeks |
Trimethoprim–sulfamethoxazole (TMP–SMX) is commonly used to treat many UTIs, except those caused by Enterococcus and Pseudomonas spp. It interferes with the bacterial metabolism of folate. TMP–SMX is highly effective and relatively inexpensive. Adverse reactions occur in 6–8% of patients using this medication; they include hypersensitivity reactions, rashes, gastrointestinal upset, leukopenia, thrombocytopenia, and photosensitivity. TMP–SMX should not be used in patients who have a folic acid deficiency state, glucose-6-phosphate dehydrogenase deficiency, or acquired immunodeficiency syndrome (AIDS), or in pregnant patients. It is the most frequently prescribed antibiotic for uncomplicated UTI (Huang and Stafford, 2002). Recently, the use of TMP–SMX has declined due to the increased incidence of bacterial resistance (Brown et al, 2002) and physicians’ preference for other newer antibiotics (Huang and Stafford, 2002). TMX can be use as monotherapy in the treatment of uncomplicated UTI without significant lost in antibacterial coverage (Nguyen et al, 2010).
Fluoroquinolones have a broad spectrum of activity, especially against gram-negative bacteria. Although they have adequate activity against Staphylococci species, fluoroquinolones do not have good activity against Streptococci species and anaerobic bacteria. They interfere with the bacterial DNA gyrase, preventing bacterial replication. Although they are highly effective in the treatment of UTI, fluoroquinolones are relatively expensive. Adverse reactions are infrequent and include mild gastrointestinal effects, dizziness, and lightheadedness. Fluoroquinolones should not be used in patients who are pregnant and should be used judiciously in children because of potential damage to developing cartilage. Due to their broad spectrum of activity, fluoroquinolones have gained popularity in the empiric treatment of both uncomplicated and complicated UTIs (Schaeffer, 2002).
Nitrofurantoin has good activity against most gram-negative bacteria (except for Pseudomonas and Proteus spp.), Staphylococci, and Enterococci species. It inhibits bacterial enzymes and DNA activity. Nitrofurantoin is highly effective in the treatment of UTI and is relatively inexpensive. Adverse reactions are relatively common and include gastrointestinal upset, peripheral polyneuropathy, and hepatotoxicity. Long-term use may result in pulmonary hypersensitivity reaction and interstitial changes. With increasing awareness of this antibiotic and its activity against common uropathogens, nitrofurantoin usage in the treatment of uncomplicated UTIs has increased from 14% to 30% in the past 5 years (Huang and Stafford, 2002).
Aminoglycosides are commonly used in the treatment of complicated UTI. They are highly effective against most gram-negative bacteria. When combined with ampicillin, they are effective against enterococci. They inhibit bacterial DNA and RNA synthesis. The principal adverse effects of aminoglycosides are nephrotoxicity and ototoxicity. Aminoglycosides are primarily used in patients with complicated UTIs who require intravenous antibiotics (Santucci and Krieger, 2000). Aminoglycosides can be given as a single daily dosing; this regimen is directed toward obtaining higher peak and lower trough levels in order to achieve more effective microbial killing while reducing toxicity (Carapetis et al, 2001).
Cephalosporins have good activity against most uropathogens (Garcia-Rodriguez and Munoz Bellido, 2000). First-generation cephalosporins have good activity against gram-positive bacteria, E. coli, and Proteus and Klebsiella spp. Second-generation cephalosporins have increased activity against anaerobes and Haemophilus influenzae. Third-generation cephalosporins have broader coverage against gram-negative bacteria but less against gram-positive bacteria. The cephalosporins inhibit bacterial cell wall synthesis. Adverse reactions include hypersensitivity and gastrointestinal upset. Oral cephalosporins have been used effectively in the empiric treatment of uncomplicated UTIs (Lawrenson and Logie, 2001); in children with febrile UTI/pyelonephritis, oral third-generation cephalosporins such as cefixime have been shown to be safe and effective (Hoberman et al, 1999).
First-generation penicillins are ineffective against most uropathogens and are not commonly used in the treatment of UTI. However, the aminopenicillins (amoxicillin and ampicillin) have good activity against Enterococci, Staphylococci, E. coli, and Proteus mirabilis. However, gram-negative bacteria can quickly develop resistance to many aminopenicillins. The addition of beta-lactamase inhibitors such as clavulanic acid makes the aminopenicillins more active against the gram-negative bacteria. Although penicillins and aminopenicillins are inexpensive, the addition of the beta-lactamase inhibitors makes them more expensive. Adverse reactions include hypersensitivity (which can be immediate or delayed), gastrointestinal upset, and diarrhea. In general, penicillins are not commonly used in the treatment of UTI unless they are combined with beta-lactamase inhibitors (Sotto et al, 2001).
The use of antibiotics has significant consequences for the treatment of future infection. Drug resistance among uropathogens has increased steadily during the past several years (Miller and Tang, 2004) and has much geographical variability. The use of broad-spectrum antibiotics has long been associated with the development of methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile superinfection. More recently, it is recognized that antibiotic use selects for and maintains antibiotic resistance as well as enhances its spread. Individuals prescribed an antibiotic in the primary care setting for a urinary infection develop bacterial resistance to that antibiotic (reviewed by Costelloe et al, 2010). The effect appears to be the greatest in the first month following treatment but may persist up to 12 months. Local hospital antibiograms, which quantifies drug resistance seen at the hospital microbiology laboratory during a particular year, can provide information regarding local antibiotic resistance among bacteria for a specific locale. Evaluating these antibiograms together, some important trends in drug resistance can be seen (Kahlmeter, 2003). Among uropathogens particularly E. coli, resistance to Ampicillin (18–54%), trimethoprim (9–27%), and sulfamethoxazole (16–49%) were high. Resistance to nitrofurantoin and fluoroquinolones were generally lower (<3%). However, with more extensive usage, resistance to these drugs is increasing (Johnson et al, 2008; Karaca et al, 2005). Even aminoglycosides that are considered to be effective, first-line choice for the treatment of complicated UTIs are not immune to the development of resistance (Lau et al, 2004). To limit the development of antibiotic resistance among uropathogens, judicial usage of antibiotics (duration and selection of the antibiotics) will be required. An uncomplicated first time cystitis does not require a 14-day course of treatment with a fluoroquinolone but simply a 3-day course of treatment with TMP–SMX.
The intestinal microbial flora consists of diverse bacterial species that inhabit the gastrointestinal tract. These bacteria are integral to the ontogeny and regulation of the immune system and protection of the body from infection. The interaction of the gut microbial flora with intestinal epithelial cells and immune cells exerts beneficial effects on the urinary tract. Intermittent and chronic antibiotic usage damages the intestinal flora. Probiotics such as lactic acid bacteria and bifidobacteria can restore normal intestinal flora and promote good host defense (Abad and Safdar, 2009). Probiotics are commonly consumed as part of fermented foods with specially added active live cultures; such as in yogurt, soy yogurt, or as dietary supplements. Their benefits remain to be definitively proven but has minimal side effects. However, probiotics should be avoided in critically ill and immunocompromised patients for the risk of sepsis.
Clinical Presentation
Acute pyelonephritis is defined as inflammation of the kidney and renal pelvis, and its diagnosis is usually made clinically.
Patients with acute pyelonephritis present with chills, fever, and costovertebral angle tenderness. They often have accompanying lower-tract symptoms such as dysuria, frequency, and urgency. Sepsis may occur, with 20–30% of all systemic sepsis resulting from a urine infection. Urinalysis commonly demonstrates the presence of WBCs and red blood cells in the urine. Leukocytosis, increased erythrocyte sedimentation, and elevated levels of C-reactive protein are commonly seen on blood analysis. Bacteria are cultured from the urine when the culture is obtained before antibiotic treatment is instituted. E. coli is the most common causative organism, accounting for 80% of the cases. Klebsiella, Proteus, Enterobacter, Pseudomonas, Serratia, and Citrobacter spp. account for the remaining cases. Of the gram-positive bacteria, Streptococcus faecalis and S. aureus can be important causes of pyelonephritis. In reproductive-age women, sexual activity and patient and family history of UTI are associated with an increased risk of developing pyelonephritis. Diabetes and urinary incontinence also independently increase this risk (Scholes et al, 2005).
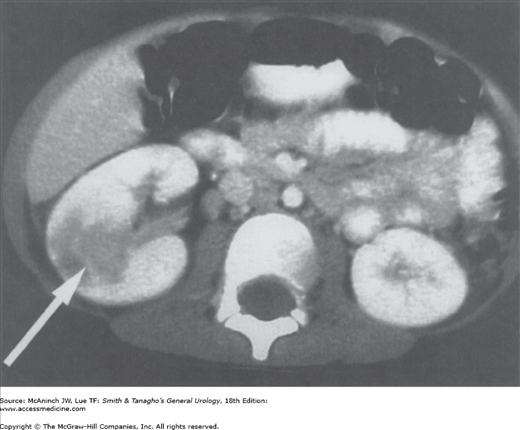
Contrast-enhanced computed tomography (CT) scans can accurately demonstrate findings, confirming the diagnosis of pyelonephritis (Dacher et al, 1993). Acute bacterial infection causes constriction of peripheral arterioles and reduces perfusion of the affected renal segments. Perfusion defects, which can be segmental, multifocal, or diffuse, are seen as areas of reduced signal density (Figure 14–2). Renal enlargement, attenuated parenchyma, and a compressed collecting system are other characteristic findings on CT scan. However, CT scan is not necessary unless the diagnosis is unclear or the patient is not responding to therapy. Radionuclide study with 99mTc-dimercaptosuccinic acid is equally sensitive in detecting the perfusion defects of pyelonephritis (Levtchenko et al, 2001). In patients with acute pyelonephritis, renal ultrasonography is important to rule out concurrent urinary tract obstruction but cannot reliably detect inflammation or infection of the kidney.
The management of acute pyelonephritis depends on the severity of the infection (Ghiro et al, 2002; Nickel, 2001). In patients who have toxicity because of associated septicemia, hospitalization is warranted. Approximately 10–30% of all adult patients with acute pyelonephritis require hospitalization, with incidence of 11.7 per 10,000 for women and 2.4 per 10,000 for men (Brown et al, 2005). Empiric therapy with intravenous ampicillin and aminoglycosides is effective against a broad range of uropathogens, including enterococci and Pseudomonas species. Alternatively, amoxicillin with clavulanic acid or a third-generation cephalosporin can be used. In a recent study of community-acquired UTIs in children hospitalized in a tertiary center (Marcus et al, 2005), it was noted that 40% of the culture-proved UTIs were caused by non–E. coli pathogens. Non–E. coli infections were more commonly found in males who had renal abnormalities and who had received antibiotic therapy in the prior month. Non–E. coli uropathogens were often resistant to cephalosporins and aminoglycosides. About 19% of the patients were initially treated with inappropriate empiric intravenous antibiotics. Fever from acute pyelonephritis may persist for several days despite appropriate therapy. Parenteral therapy should be maintained until the patient defervesces. If bacteremia is present, parenteral therapy should be continued for an additional 7–10 days and then the patient should be switched to oral treatment for 10–14 days. In patients who are not severely ill, outpatient treatment with oral antibiotics is appropriate. For adults, treatment with fluoroquinolones or TMP–SMX is well tolerated and effective. Therapy should continue for 10–14 days. Some patients in whom acute pyelonephritis develops will require follow-up radiologic examination such as voiding cystourethrogram or cystoscopy.
Emphysematous pyelonephritis is a necrotizing infection characterized by the presence of gas within the renal parenchyma or perinephric tissue. About 80–90% of patients with emphysematous pyelonephritis have diabetes; the rest of the cases are associated with urinary tract obstruction from calculi or papillary necrosis (Shokeir et al, 1997; Tseng et al, 2005).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree