Chapter 38 Assisted Reproductive Technology: Clinical Aspects
INTRODUCTION
In vitro fertilization (IVF) is a remarkable scientific approach to the common clinical problem of infertility. The initial development of IVF in humans can be attributed directly to a team of two investigators, Drs. Patrick Steptoe and Robert Edwards. It was in 1969 that Dr. Edwards first announced, “Human oocytes have been matured and fertilized by spermatozoa in vitro. There may be certain clinical and scientific uses for human eggs fertilized by this procedure.”1 This understated conclusion marked the first successful attempt to fertilize human eggs in a laboratory.
Currently, more than 100,000 cycles of human IVF and similar techniques are performed each year in the United States, resulting in the birth of more than 40,000 babies. IVF, together with the much less commonly used techniques of gamete intrafallopian transfer (GIFT) and zygote intrafallopian transfer (ZIFT), are collectively referred to as assisted reproductive technologies (ART). Today, ART procedures are responsible for approximately 1% of all children born in the United States annually.2
Assisted Reproductive Technology Techniques
In Vitro Fertilization
In 1891, the first successful transfer of an embryo from one animal to another that resulted in birth was reported, using rabbits of two different strains. However, these embryos were obtained from eggs fertilized in vivo. Further progress toward the goal of IVF was slowed because of limited understanding of the maturation of eggs and sperm required to achieve fertilization and embryo development. In 1959, successful IVF was reported using rabbits.3
The first human birth to result from IVF was achieved in England in 1978.4 John and Lesley Brown had 9 years of infertility secondary to bilateral fallopian tube obstruction. Dr. Patrick Steptoe surgically retrieved a single mature oocyte from one of Lesley’s ovaries during a natural cycle. Dr. Robert G. Edwards combined John’s sperm with the oocyte in the laboratory and the resulting embryo was placed into Lesley’s uterus a few days later. On July 25, 1978, Louise Joy Brown was delivered by cesarean section at approximately 37 weeks’ gestation, and weighed 5 pounds 12 ounces.
Gamete Intrafallopian Transfer
Although early reports of GIFT indicated higher success rates than with IVF, improvements in the formulation of culture media and other laboratory techniques have resulted in equivalent success rates for GIFT and IVF.5,6 A disadvantage of GIFT is that it can only be performed in patients with normal fallopian tubes. Another disadvantage of GIFT compared to IVF is that it generally requires laparoscopy, with all the risks and expenses associated with this outpatient surgery. Currently, GIFT procedures account for less than 0.2% of ART treatments in the United States.
Zygote Intrafallopian Transfer
Although some studies comparing ZIFT to conventional IVF have found ZIFT to be superior, other studies have shown little difference.7,8 This uncertainty and the need for two surgical procedures for ZIFT (i.e., transvaginal egg retrieval and laparoscopic embryo transfer) have limited the popularity of this technique. However, ZIFT has found a place in the treatment of infertile women with congenital or acquired cervical abnormalities and in patients who fail to achieve pregnancy after repeated IVF cycles.9,10 Currently, ZIFT procedures account for approximately 0.5% of ART treatments in the United States.
INDICATIONS FOR ASSISTED REPRODUCTION
Tubal Factor Infertility
Tubal Adhesions
Tubal adhesions account for 30% to 40% of cases of female infertility. Tubal damage or adnexal adhesion typically arise from salpingitis, appendicitis, endometriosis, or previous pelvic surgery. Laparoscopic surgery to remove adhesions and open tubes can yield pregnancy rates of more than 50% in women with relatively mild disease.11 In women with severe tubal damage or extensive dense adhesions, surgery is unlikely to result in pregnancy. IVF is indicated in these patients and in women who have failed to conceive after infertility surgery.
Hydrosalpinges
Tubal surgery is also indicated in women with hydrosalpinges who are contemplating IVF. Hydrosalpinges are associated with decreased pregnancy and live birth rates after IVF.12 Although the pathophysiology of this relationship is not completely understood, bilateral salpingectomy improves the success of subsequent IVF, especially in women with bilateral hydrosalpinges.
Tubal Ligation
Up to 25% of women who have undergone bilateral tubal sterilization will come to regret their decision and wish to have more children. Risk factors for tubal ligation regret include young age at time of sterilization, relationship with a new partner, loss of a child, and having been sterilized immediately postpartum or postabortion. Up to 5% will seek tubal anastomosis, which is a highly effective treatment option in properly selected patients.13 Success rates depend on the site of the anastomosis, the length of residual tube, the age of the patient, and the presence of other infertility causes.
Endometriosis
Endometriosis is a common cause of both infertility and pain (see Chapter 49). The effects of endometriosis on fertility can be decreased but not completely circumvented by the combination of gonadotropin stimulation and intrauterine insemination.14 The decrease in monthly fecundibility roughly correlates with the severity of disease, although there is a poor correlation between endometriosis stage and the chance of pregnancy after surgical treatment.15
In vitro fertilization is an effective treatment for infertile women with endometriosis who fail to conceive with less aggressive treatment. Some, but not all, studies suggest that endometriosis affects IVF success. Endometriosis has been implicated in poor ovarian reserve, poor quality of oocytes and embryos, and poor implantation.16–21 Prolonged hormonal suppression using gonadotropin-releasing hormone (GnRH) analogues appears to improve IVF success in women with endometriosis.22
Male Factor Infertility
At least 40% of men who are members of infertile couples have abnormal semen analyses.23 Achieving pregnancy via conventional IVF in oligospermic men met with disappointing results largely due to fertilization failure.24 The treatment of male infertility dramatically improved with the development of intracytoplasmic sperm injection (ICSI).25 Injection of a single sperm into the egg appears to solve fertilization failure for the majority of male infertility problems. Currently, ICSI is used in about half of all ART treatment cycles. This technique is discussed in more detail in Chapter 39.
Antisperm Antibodies
Antisperm antibodies are a relatively uncommon and difficult to treat cause of infertility. IVF with ICSI has been found to be an effective treatment for women with antisperm antibodies, even in patients with a higher density of such antibodies.26 In one study, patients with antisperm antibodies had a 32% clinical pregnancy rate after IVF with ICSI.27 Because antisperm antibodies are relatively uncommon, it does not appear that routine screening for antisperm antibodies before IVF is cost-effective.28
Unexplained Infertility
Up to 30% of infertile couples will have unexplained infertility.29 Treatment options for unexplained infertility include ovarian stimulation with clomiphene citrate or gonadotropins, plus intrauterine insemination. IVF is an effective treatment for couples with unexplained infertility who fail to conceive with these approaches. The success of IVF in couples with unexplained infertility appears to be comparable to that achieved in cases of tubal damage or endometriosis.24
Failure to Conceive After Ovulation Induction
Anovulatory women who fail to conceive with ovulation induction are good candidates for IVF. Unfortunately, the pregnancy rates after IVF are lower and the complication rates higher for patients with polycystic ovary syndrome (PCOS). In one study of 110 women with PCOS who underwent IVF/ICSI, women with a body mass index (BMI) greater than 29 kg/m2 had a lower pregnancy rate per oocyte retrieval and a higher occurrence of ovarian hyperstimulation syndrome as compared to PCOS patients with a BMI of 29 kg/m2 or lower.30 Treatment with metformin to lower insulin levels in patients with PCOS undergoing IVF has been reported to improve success rates.31
Diethylstilbestrol Exposure
Women exposed in utero to diethylstilbestrol (DES) are known to have an increased risk of infertility.32 These women are also at increased risk of pregnancy complications as a result of reproductive tract abnormalities such as a T-shaped or hypoplastic cavity, a septate uterus, or uterine synechiae.33 IVF outcomes in DES-exposed women are comparable with respect to ovarian response and embryo quality, but delivery rates are lower, possibly due to uterine abnormalities.33
PATIENT SELECTION—PREDICTORS OF SUCCESS FOR IVF
In theory, only three things are needed to accomplish a successful IVF cycle: eggs, sperm, and a uterus into which the embryos are transferred. Although fertility testing is covered in Chapter 34 and Chapter 35, certain aspects of the evaluation specific to ART treatment follow.
Evaluation of the Uterus
Endometrial Polyps
Endometrial polyps, benign localized overgrowths of endometrial tissue of uncertain etiology, are present in up to 10% of asymptomatic premenopausal women over age 30.34 It is assumed that endometrial polyps decrease fertility. For this reason, all large polyps are removed prior to IVF.
For smaller polyps that first appear during ovarian stimulation for IVF, optimal management remains to be determined. The problem is that removal requires interruption of the cycle and delay of IVF. A series of 49 women with endometrial polyps less than 2 cm in diameter who underwent IVF without removing the polyp found pregnancy and miscarriage rates were comparable to the general pregnancy rate of their IVF clinic population, although there was a trend toward a higher miscarriage rate.35 At the present time, the decision to continue despite the polyp or stop the cycle and remove the polyp must be made on a case-by-case basis.
Hydrosalpinges
The presence of hydrosalpinges is well documented to decrease the success rate for IVF.36 Whether pregnancy rates can be improved by salpingectomy remains less certain. A recent meta-analysis of three randomized, controlled trials indicated that the chance of live birth with IVF was doubled by pretreatment salpingectomy.12 This effect seems to be most apparent in women with bilateral hydrosalpinges and with hydrosalpinges that are sonographically visible.37 Drainage of hydrosalpinges at the time of egg retrieval can be performed, but it is uncertain if this improves IVF pregnancy rates. At present, many IVF programs offer patients with a sonographically visible hydrosalpinx the option of pretreatment salpingectomy.
Evaluation of the Ovaries
Age
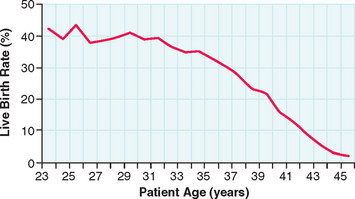
It has long been known that reproductive capacity declines with increasing age.38 The age-dependent decline in female fertility can be partly attributed to the fact that women have a finite and nonreplenishable number of germ cells. The peak number of germ cells occurs at midgestation during fetal life and declines continuously thereafter, with an accelerated loss of oocytes between ages 37 and 38.39 Diminished ovarian reserve is a term used to indicate decline in reproductive capacity associated with ovarian follicular depletion and diminished oocyte quality.
The success of IVF declines with age in a similar fashion (Fig. 38-1). For women undergoing IVF, diminished ovarian reserve is associated with poor ovarian response to gonadotropins, cycle cancellation, and lower chances of conception. Despite the known correlation with chronologic age and ovarian reserve, there exists a tremendous amount of variability in patients. As a result, multiple markers of ovarian reserve have been sought to supplement age as a predictor of ovarian response to stimulation in IVF. Timely recognition of reduced ovarian reserve is important for counseling patients prior to IVF.
Basal FSH
A direct correlation of the basal FSH and IVF outcome measurements was found in a study of 441 patients undergoing 758 consecutive IVF cycles.40 Patients with basal FSH levels greater than 25 mIU/mL had only a 3.6% ongoing pregnancy rate, whereas those with basal FSH levels less than 15 mIU/mL had a 17% pregnancy rate. They also noted that fewer follicles were aspirated, fewer oocytes were obtained, and fewer embryos were available for transfer in the high FSH group compared to the low FSH group.
Day 3 Estradiol
Serum estradiol levels on day 3 of the menstrual cycle have also been found to be predictive of subsequent IVF pregnancy rates. One study found that the ongoing pregnancy rates for patients with day 3 estradiol levels less than 30 pg/mL were significantly higher than for patients with estradiol levels between 31 and 75 pg/mL.41 No pregnancies occurred in patients with day 3 estradiol levels greater than 75 pg/mL. Another study found that the 97.5th percentile predictive value for day 3 estradiol for pregnancy was 56 pg/mL.
Basal elevations of estradiol are an independent marker of poor ovarian response to stimulation even in cycles without a rise in FSH. This is presumably because high circulating levels of serum estradiol suppress FSH levels. This hypothesis was confirmed in a study of 225 patients, where no pregnancies occurred after IVF with day 3 estradiol greater than 100 pg/mL, despite FSH levels less than 15 mIU/mL in all patients.42 In a study of 2476 IVF patients who had normal day 3 FSH levels, patients with day 3 estradiol levels either less than 20 pg/mL or greater than 80 pg/mL had an increased cancellation rate.43 However, estradiol levels are no longer predictive of IVF pregnancy rates once the patients had more than three maturing follicles.
Inhibin B
Elevation of FSH occurs in part as a result of diminished inhibin secretion by developing follicles. It follows that inhibin levels may also predict IVF success. Inhibin is a heterodimer that is secreted in two forms: inhibin A and inhibin B. In one IVF study, women with a day 3 serum inhibin B concentration less than 45 pg/mL had lower estradiol levels, fewer oocytes retrieved, a higher cycle cancellation rate, and a lower clinical pregnancy rate than women whose inhibin B concentration was at least 45 pg/mL.44 However, another study of 120 women undergoing IVF found that inhibin B levels did not improve the prediction of IVF success over using patient age and basal FSH level.
Antimüllerian Hormone
Antimüllerian hormone is produced by developing granulosa cells. A recent study of 130 women undergoing their first IVF cycle found that low antimüullerian hormone levels were associated with fewer eggs retrieved and increased chance of cycle cancellation.45 According to another study, basal serum antimüllerian hormone level may be a better predictor of IVF outcome than FSH, estradiol, or inhibin levels.46
Antral Follicle Count
The number of small follicles visible by transvaginal ultrasonography early in the follicular phase is another means used to predict ovarian response in IVF. A prospective IVF study of 130 women younger than age 45 indicated that the number of antral follicles on cycle day 3 provided better prognostic information regarding poor ovarian response during hormone stimulation for IVF than age or basal FSH, estradiol, or inhibin B levels. Another IVF study of 120 women also found that a single antral follicle count was predictive of poor ovarian response, although the predictive accuracy could be increased by repeating the count on a subsequent cycle and using the higher of the two counts.47 The exact number of antral follicles that predicts outcome is still uncertain.
Clomiphene Citrate Challenge Test
The CCCT decribed by Navot and colleagues is a method to dynamically elucidate the ovarian response.48 The test is performed by measuring basal FSH on day 3 (day 2 is acceptable), administering clomiphene citrate (100 mg, days 5 to 9), and then remeasuring a FSH on day 10 (days 9 and 11 are also acceptable). An abnormal test was originally defined as an FSH level after clomiphene citrate more than 2 standard deviations from the basal level. Many clinicians define an abnormal CCCT as an FSH value greater than 12 mIU/mL on either cycle day 3 or 10.
The CCCT has been shown to have a sensitivity of 43% and a specificity of 76%, using IVF cycle cancellation as an endpoint in a study of 198 women.49 Positive and negative predictive values were 37% and 80%, respectively. The estradiol levels during ovarian stimulation, the number of retrieved oocytes, and the rate of transfer cycles were significantly lower in patients with an abnormal CCCT. Forty-three percent of the abnormal test results were abnormal only on their elevation of day 10 or 11 FSH and not on their basal FSH level. However, the rate of pregnancies per started cycle did not show a statistically significant difference, which was attributed to the low numbers of patients.
A recent meta-analysis of a total of 1352 patients from 12 studies on basal FSH and 7 studies on CCCT found that basal FSH had a sensitivity of 6.6% and a specificity of 99.6% for identifying inability to achieve pregnancy in an IVF cycle, whereas CCCT sensitivity was 25.9% and specificity was 98.1%.50 This study suggests that basal FSH and CCCT are similar in the ability to predict a clinical pregnancy and that, although a normal test is not helpful, an abnormal test is highly predictive that pregnancy will not occur with IVF. Based on this, the authors recommended that basal FSH be used rather than CCCT because of its simplicity and lower cost.
Combining Screening Markers
It is apparent that there are clear limitations to each of the proposed markers for ovarian response to stimulation. Therefore, attempts have been made to combine some of these markers to improve the predictive ability compared to a single marker alone. When basal FSH and estradiol are combined for patients undergoing IVF cycles, one study reported no pregnancies in patients with basal FSH levels greater than 17 mIU/mL and basal estradiol level greater than 45 pg/mL.41 Another study of 74 IVF patients with basal FSH levels less than 15 mIU/mL examined the ratio of basal FSH to LH.51 This study found that an elevated FSH-to-LH ratio greater than 3.6 correlated with a lower day 8 estradiol, lower peak estradiol, and fewer follicles greater than 15 mm in size
A randomized study of 110 patients evaluated multiple markers for ovarian reserve, including the exogenous FSH ovarian reserve test. This test involves the administration of 300 IU of rFSH (Gonal-F) on cycle day 3 with serum measurements of FSH, estradiol, and inhibin B before and 24 hours after administration. They concluded that the exogenous FSH ovarian reserve test was better than the CCCT at predicting the number of large follicles resulting from controlled ovarian hyperstimulation.52
OVARIAN STIMULATION FOR IVF
Clomiphene Citrate
Wood and colleagues were the first to report that administration of clomiphene citrate followed by human chorionic gonadotropin (hCG) to complete oocyte maturation both increased IVF success rates and improved scheduling efficiency for egg retrieval.53 Clomiphene citrate was commonly used by early IVF programs in the United States until higher success rates were reported using human menopausal gonadotropins.
Clomiphene citrate is used at an oral dose of 50 to 150 mg/day for 5 days beginning on day 2 or 3 of the menstrual cycle, and hCG (5,000 to 10,000 units intramuscular) is given when the lead follicle reaches 18 mm in diameter. Although gonadotropins remain the standard ovarian stimulants for IVF, the convenience and low cost of clomiphene has caused it to be reconsidered as an option for good-prognosis IVF patients.54–56
Gonadotropins
The use of the injectable gonadotropin FSH, with or without LH, circumvents the natural decline of FSH that occurs with development of the dominant follicle. In effect this rescues oocytes that would be physiologically lost to atresia in a natural cycle, which selects only one dominant follicle. Initial reports in the United States using human menopausal gonadotropins (a combination of FSH and LH) for IVF were considered spectacular, with pregnancies achieved in 5 of 24 laparoscopic egg retrievals (21%), at a time when IVF pregnancy rates after other stimulation protocols were less than 10%.57 Although initial success likely was due in part to improvements in laboratory techniques and patient selection, gonadotropin injections soon became the standard treatment to prepare women for egg retrieval.
Human chorionic gonadotropin 5,000 to 10,000 units is typically used to mimic the LH surge and complete oocyte maturation in gonadotropin cycles. Egg retrieval is performed 34 to 36 hours after the hCG injection. Urinary and recombinant hCG products appear to give equivalent results.58
There has been much investigation and discussion of the relative merits of different gonadotropin preparations.59,60 Most programs in the United States have gravitated to some combination of FSH and LH for ovarian stimulation, along with a GnRH analogue. Recombinant and urinary FSH products seem to give equivalent pregnancy rates.61 Adjunctive stimulation with clomiphene in gonadotropin cycles, while lowering total gonadotropin doses required, has fallen out of favor because of the risk of spontaneous ovulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree