Fig. 5.1
AMS 800 artificial urinary sphincter (courtesy of AMS)
TheAMS 800 is instilled with an antibiotic coating of InhibiZone, which contains minocycline and rifampin. Studies related to effectiveness have generally been in the setting of other genitourinary prosthetic surgery, specifically inflatable penile prosthesis (IPP). Carson showed a decreased risk of infection complications for IPP treated with InhibiZone compared to controls [3], which was corroborated in other studies [4]. However, studies specifically in AUS have not demonstrated an obvious benefit from the antibiotic coating in terms of infectious complications, though the coating is associated with increased costs [5]. Nonetheless, InhibiZone remains the standard antibiotic coating on AMS 800, though non-coated devices are available. When using antibiotic coated devices, they may be externally rinsed occasionally with fluid, but they should not be soaked in saline as this can dilute its concentration. Of note, the use ofInhibiZone is contraindicated in patients with known allergy to tetracyclines or rifampin. Additionally, InhibiZone is contraindicated for those patients with systemic lupus erythematosus as minocycline has been reported to exacerbate the disease [6].
Patient Selection
History and Physical Exam
When considering placement of the artificial urinary sphincter, a successful outcome begins in the clinic. While urologists have some surgical options for the treatment of incontinence, many patients are not appropriate candidates for one or more therapies. Estimates of incontinence after prostatectomy vary based on series as well as the definition of incontinence used, but multiple series report a 7–16 % incidence of some level of leakage [7–9]. Patients with other causes of stress incontinence are candidates for AUS, including those with spinal cord injuries and women with intrinsic sphincter deficiency.
A thorough urologic history is important to ascertain and should identify the cause of incontinence. The surgeon should be careful to elicit any other priorabdominopelvic surgeries that may complicate reservoir placement. The degree of incontinence, usually in terms of the numbers of pads used daily and the degree to which they are soaked, should also be evaluated as alternative treatments are reasonable for less severe incontinence [10]. Some men with mild leakage may elect to undergo sling placement or injection of bulking agents. Of note, the number of pads alone has been thought to be unreliable as there is considerable variation in the amount of urine leakage tolerable by men before absorbent pads are changed. Some urologists, in an effort to be more accurate, may utilize other instruments, such as pad weight. In theSUFU Pad Test Study , Nitti et al. demonstrated that the subjective patient-perceived amount of leakage actually correlated well with the true amount of leakage by weighing pads over 24 h [11]. Utilizing newer technology, Pepper et al. demonstrated the feasibility and usefulness of a mobile app to document urinary incontinence symptoms [12]. Such an approach can be used as an alternative to the traditional bladder diary or patient recall. No matter the approach, understanding the degree of leakage with a patient preoperatively can help to realistically set expectations of dryness after any planned procedure.
Identifying those patients with primarily urge incontinence remains an important step before AUS placement as implantation of the device may exacerbate rather than relieve symptoms of urgency. As an elevated detrusor leak point pressure of over 40 cm water has been shown to be associated with upper tract deterioration [13, 14], increasing outflow resistance in the setting of a “hostile” bladder is a risk and may lead to renal deterioration. In the setting of prior radiotherapy, Gomha and Boone reported similar outcomes of AUS for post-prostatectomy incontinence compared to those without radiation though urinary urgency was notably high (44–47 %) in both groups [15]. Additionally, Ravier et al. reported similar functional outcomes but increased rate of major complications for AUS placement in patients with prior radiation [16]. A largemulticenter prospective study of 386 patients demonstrated that radiation, prior AUS erosion and history of urethral stent increased the risk of AUS explantation [17]. Taken in total, the risks and benefits should be carefully assessed before any surgical undertaking in these higher risk patients, though AUS placement certainly is feasible and usually met with excellent outcomes.
Understanding a patient’s wishes and expectations is another critical preoperative step. Those with mild urinary incontinence may prefer to live with their symptoms rather than pursue AUS placement given its associated morbidity and risk. Additionally, other surgical procedures, such as sling placement or injection of bulking agents, may be viable alternative therapies for certain patients. Given that a significant portion of the psychosocial impact associated with stress urinary incontinence is related to the quantity of loss of urine [18], a reasonable nonsurgical option to prevent leakage is the use of an external Cunningham clamp, a device that has been shown to outperform other penile compression devices [19]. With an AUS, there is some concern about the need to manage a mechanical device in order to urinate, which is one advantage of using the male sling. In fact, Kumar et al. reported that when men with post-prostatectomy incontinence were offered a choice of AUS and male sling, 92 % of patients chose the sling in order to avoid using a mechanical device [20].
Manual dexterity and patient understanding should be assessed prior to surgical intervention. Adequate use of an AUS requires regular and reliable fine motor skills to manipulate the pump, which is typically located in the dependent portion of the scrotum. While the exact cutoff for the minimum dexterity needed is nebulous, one should exercise caution if considering AUS placement in patients with advanced age or a progressive neurological disease. Although caretakers can manage the artificial urinary sphincter if necessary, relying on others in these situations is generally precarious. As patients age, the device can be deactivated if the patient cannot manage the pump, but this would lead to recurrent incontinence. As the age of the population continues to rise, additional patients and their caregivers will likely be faced with these situations. While further study is needed to ascertain the safest course, communication between patient and family members is key while patients are independent and active. This may help prevent, for example, inadvertent catheterization with an activated AUS leading to increased morbidity for patients unable to fully participate in their own healthcare related decisions. Advising patients to consider the benefits of a medical ID bracelet may mitigate some of these issues.
A general medical history should also be elicited from the patient. Given the consequences of infection, patients at higher risk such as those with diabetics or who are immunocompromised should be identified and counseled carefully.Hyperglycemia in the perioperative period for various surgeries has been associated with longer hospital stay, higher health care resource utilization and greater perioperative mortality [21]. It remains controversial if tight glycemic control in the perioperative and postoperative period is beneficial, but certainly conventional glycemic control is generally considered the current standard [22]. Those patients with a history of prior implant infection, evidence of frequent unexplained infections or an immunocompromised state may benefit from infectious disease consult prior to consideration of AUS implantation. Patients with rheumatoid arthritis or inflammatory bowel disease on immunomodulators may consider a drug holiday in partnership with their prescribing physician. In summary, these special populations should be appropriately counseled regarding the potential increased morbidity and mortality associated with implantation surgery.
Erectile dysfunction , which is relatively more common than incontinence after prostatectomy, should be evaluated during the clinic visit. While there are numerous medical therapies for ED, if it remains refractory, the most definitive surgical treatment is penile prosthesis. While some surgeons have reservations regarding simultaneous AUS and penile prosthesis placement given possible increased risk of infection, multiple studies have demonstrated that it is safe with similar perioperative and subjective outcomes [23, 24].
Performing an appropriate physical exam is an important step before incontinence surgery. While likely identified during thorough history taking, it is important to note any prior surgical incisions or abnormal anatomy, which can be critical for operative planning. For example, noting that a minimally invasive (typically transperitoneal) or open (typically extraperitoneal) approach had been undertaken for previous radical prostatectomy may help the surgeon in determining reservoir placement. Additionally, the patient should be checked for an inguinal hernia, which may require repair or change the expected location of the reservoir. Alternative causes of incontinence can be evaluated or ruled out by testing for perineal sensation, bulbocavernosus reflex or rectal exam. Additionally, it is important to examine the operative area to ensure there are no active skin infections or anatomic abnormalities that would preclude surgery. An informal assessment of a patient’s digital manipulation skills can usually be performed passively during an encounter, but if any tremor or weakness is suspected, a full neurologic exam or referral would be appropriate. In a female, a thorough pelvic exam is necessary to evaluate for Valsalva-induced leakage before undergoing any surgical interventions for stress incontinence.
Preoperative Testing
An incontinence evaluation generally includes urine studies, a voiding diary and may also include an assessment of post-void residual. Cystoscopy is often performed prior to surgery to delineate anatomy and rule out stricture disease. Post-prostatectomy patients with incontinence should be evaluated for bladder neck contracture. Studies have estimated the incidence of bladder neck contracture at 1.1–1.4 % after robotic prostatectomy [25, 26], which seems to be slightly lower than the reported rates of 2.5–2.6 % for open retropubic prostatectomy [26, 27]. Any stricture or bladder neck contracture should be treated and monitored for recurrence prior to surgery. AUS in patients with history of prior urethral stricture should be performed with great caution as endoscopic treatments, such as urethrotomy for simple urethral strictures, are associated with a high recurrence rate with a median time to recurrence of 9 months [28].
Prior to AUS placement, a few basic laboratory studies should be performed. A basic metabolic panel and complete blood count assess for renal function, leukocytosis, and thrombocytopenia. If diabetic, a hemoglobin A1C can assist in ascertaining the patient’s 3-month glucose control. Poorly controlled diabetics are at a greater risk for infectious complications. In addition, routine urinalysis and/or culture should be performed to rule out infection or bacteriuria, both of which should be treated prior to surgery.
Urodynamic studies (UDS) prior to AUS placement are a matter of some debate. Certainly if needed to confirm the diagnosis or rule out alternative etiologies, UDS can be of considerable utility. In the setting of post-prostatectomy incontinence, preoperative urodynamics is generally left to surgeon preference. While detrusor underactivity is relatively common after radical prostatectomy [29], multiple studies have demonstrated that adverse preoperative urodynamic features do not negatively affect continence results after AUS placement [30, 31]. If not undergoing preoperative cystoscopy, urodynamics can add useful anatomic information. For example, in a study of 169 men with post-prostatectomy incontinence, 32 men (19 %) did not have demonstrable incontinence while the UDS catheter was in place but had leakage after the catheter was removed. Of these 32 patients, 18 men (56 %) were found to have an anastomotic stricture [ 32].
Considerations with Prior Incontinence Surgery
For patients with prior incontinence surgery, surgeons should pursue a thorough preoperative workup. Similar to the workup for a patient without prior incontinence surgery, ascertaining the degree of leakage is key. A complete history regarding timing and duration of failure may hint at the etiology. For example, the patient may simply not be operating the AUS correctly so the exact technique should be demonstrated by the patient and observed by the physician. Cystoscopy is mandatory in the setting of a prior AUS to evaluate for erosion, mechanical failure, stricture and urethral atrophy. While visualizing the area of the artificial sphincter, the device should be cycled. If the device is confirmed to be working appropriately, then alternative diagnoses should be entertained. Urethral atrophy can be identified in this manner. If the device does not cycle, a leak of filling solution may have occurred. This can be determined by performing an abdominal ultrasound or if a filling solution of diluted contrast was used during the initial procedure, an abdominal X-ray can identify an appropriately filled reservoir. In the setting of a functional, appropriately managed AUS, urodynamic studies may reveal adverse findings such as detrusor overactivity or poor compliance, which may identify a treatable etiology and prevent unnecessary surgical intervention.
Urethral atrophy is a common cause of recurrent incontinence after AUS. There is some controversy regarding the optimal management. The options include cuff downsizing [33], relocation of the cuff proximally [34], transcorporal placement [35], or use of a tandem cuff [36]. Comparative studies are unfortunately lacking. Therefore, ultimately, treatment is decided based on anatomic considerations and surgeon preference. Regardless, large series have shown favorable results for secondary sphincter implantation compared to primary AUS placement [37].
It is important to remember that recurrent incontinence due to mechanical malfunction of an indwelling artificial urinary sphincter does not obligate surgical intervention. If no erosion or infection is discovered, it is ultimately a decision made between the patient and surgeon. If improving the patient’s incontinence, likely in the form of complete device replacement, is worth the known risks and complications then reoperation should occur. At the very least, device malfunction is not an emergency and careful decision making and operative planning can commence prior to surgical revision.
Practice Models, Patterns and Learning Curve
The management of patients with post-prostatectomy incontinence remains non-standardized, and male incontinence surgery is generally underutilized. This may be secondary to feelings of embarrassment, belief that treatment is futile or lack of knowledge regarding management options. Reynolds et al. demonstrated considerable state and regional variation in the use of AUS even when controlling for differences in rates of prostatectomy and distribution of urologists, suggesting underutilization in certain areas of the country [38]. Similarly, considerable variation in the performance of AUS has been demonstrated on an international level [39]. Even though the overall number of incontinence procedures has steadily risen with time [40], only a small minority of surgeons perform a high volume of artificial urinary sphincter cases. Additionally, Kim et al. reported that in a population based cohort of older men, only 6 % of men underwent an incontinence procedure after prostatectomy [41]. Multiple issues may be playing a role. In addition to patient factors such as patient perception or misconception, surgeon availability, and expertise to perform the procedure may also limit AUS placement in otherwise viable operative candidates.
The learning curve for the placement of artificial urinary sphincter remains controversial. While one single surgeon study suggested a learning curve of AUS was roughly 25 cases [42], a larger study with multiple surgeons demonstrated a slow but steady decrease in reoperative rates showing no plateau through 200 procedures, indicating a prolonged learning curve [43]. The majority of patients were treated by surgeons who had performed a total of ≤25 AUS placements with only 9 % seeing a surgeon with ≥100 prior procedures [43]. Similarly, Lee et al. reported that over 90 % of AUS cases are done by surgeons who performed five or fewer each year [44]. As a result, there may be considerable room for preventable cases of reoperation for this reconstructive surgery. One study demonstrated the feasibility of a formal regional referral service for post-prostatectomy incontinence, which can lead to standardized, high level care from specialized urologic centers [45].
Surgical Technique
Preparation
Various steps should be taken prior to artificial urinary sphincter insertion. While ruling out urinary or cutaneous infections is imperative before incontinence surgery, many surgeons choose to prescribe a topical home antimicrobial regimen before the patients arrive in the hospital. In fact, employing a chlorhexidine based 5-day preoperative scrub has been shown to decrease bacterial skin colonization compared to traditional soap and water for patients undergoing AUS insertion [46].
On the day of surgery, hair removal is necessary prior to skin cleaning and incision. A Cochrane review regarding preoperative hair removal concluded that clippers were associated with fewer surgical site infections than shaving with a razor [47]. These recommendations should be followed on the day of surgery to minimize infectious risks.
Intravenous prophylactic antibiotics should be given prior to skin incision. Various recommendations (such as vancomycin with gentamicin or cephalosporin with gentamicin) have been published regarding ideal antibiotic choice prior to genitourinary prosthetic surgery [48]. The regimen should generally garner broad antimicrobial coverage against skin and urinary organisms and can be modified in regard to local resistance patterns and patient allergies. Intravenous antibiotics are generally continued for 24 h, and many surgeons choose to keep patients on some oral antimicrobial for up to 1 week after discharge. Extended duration prophylaxis (>24 h) in this setting has not been well studied and should be approached with some caution given the theoretical, though admittedly low, risks of adverse events such as resistant bacteria or Clostridium difficile colitis. As mentioned previously, special populations, such as patients with history of previous prosthetic infections, patients who are immunocompromised or those on immunomodulating drugs, may benefit from infectious disease consultation to assist in antibiotic selection and duration.
Preoperative antimicrobial topical surgical site preparation is critical in establishing a sterile operative field. While choice of cleansing solution varies in surgical practice, a randomized controlled trial has shown chlorhexidine–alcohol to be superior to povidone–iodine in terms of superficial and deep incisional infections for clean-contaminated cases [49]. Specifically for genitourinary prosthetic surgery, chlorhexidine–alcohol has also been shown to be superior to povidone–iodine in terms of eliminating skin flora based on preoperative and postoperative cultures [50]. Some authors recommend an extended time for surgical scrubbing (10–15 min). Though this has not been well studied, there would seem to be little downside other than the few additional minutes of anesthesia.
AUS in the Bulbar Urethra
If not performed preoperatively, cystoscopy may be performed at the start of the procedure to rule out stricture disease. A 14 French Foley catheter is inserted on the sterile field, which facilitates identification and dissection of the urethra as well as possibly providing standardized sizing while choosing the appropriately sized cuff. The bladder should be drained for reservoir placement. Although urine is theoretically sterile, urine contamination into the operative field can be limited by utilizing gravity bag drainage as opposed to the suction tip, which will be used later in the operative field.
Incision and Urethral Dissection
The usual location of the incision is the perineum just inferior to the scrotum. A midline, longitudinal incision is made over the urethra, which can be palpated with the urethral catheter in place. The incision is extended 4–6 cm but should not approach the anus, which is ubiquitously colonized with bacteria and should be excluded by the surgical drapes. A marking skin staple can be used to ensure the surgeon is aware of the most caudal extent of the operative field. A Turner-Warwick retractor can be used to optimize exposure of relevant anatomy. Electrocautery is used to minimize bleeding in the operative field until the bulbospongiosus is encountered. The surgeon should switch to sharp dissection with Metzenbaum scissors to divide the bulbospongiosus to visualize the urethra. Great care should be taken during urethral dissection. Generally, blunt dissection or “spreading” should be avoided when mobilizing the dorsal aspect of the urethra. Instead, controlled sharp dissection using Metzenbaum scissors, with the urethra under lateral tension, is preferred to minimize the risk of urethral laceration.
The location of the cuff has been historically in one of two positions. For post-prostatectomy patients, the location is typically the bulbar urethra (Fig. 5.2). Surgical dissection for bulbar urethral placement is relatively straightforward using the perineal approach. However, an upper transverse transscrotal approach remains an alternative with the advantage of placing the reservoir through the same incision [51]. There is some evidence that the perineal approach provides superior outcomes compared to a transscrotal approach [52]. The bladder neck is the alternative location, which is typically used in women and children, and is also often preferred in men with an intact prostate (Fig. 5.3). Of note, a bladder neck cuff is contraindicated for post prostatectomy incontinence. For men, it is generally reserved for patients with neurologic etiologies of incontinence such as neurogenic bladder or exstrophy. Dissection of the urethra is more challenging for bladder neck placement as it requires circumferential dissection of the urethra at the junction of the bladder and prostate. If bladder neck placement is desired for the cuff, a variety of alternative surgical approaches may be pursued. For example, studies have shown satisfactory results with a number of approaches including abdominal [53], vaginal [54], purely laparoscopic [55], and robotic techniques in both men [56] and women [57, 58].
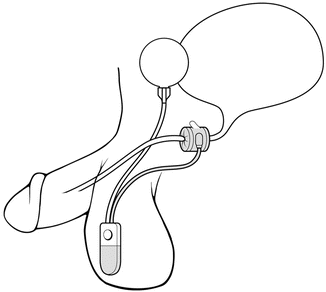
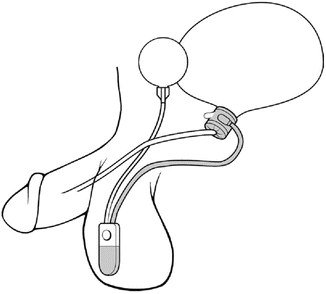

Fig. 5.2
Bulbar urethra placement of the AUS (courtesy of AMS)

Fig. 5.3
Bladder neck placement of AUS (courtesy of AMS)
Device Preparation
Careful technique should be used during preparation of the various system components of the AUS. This ensures that free air is not introduced into the system, which can cause malfunction due to lockout. The first step involves preparation of the control pump. The two ends of the control pump tubing are placing in a basin with filling solution. The pump is repeatedly squeezed while keeping the tips of the tubing submerged and the tubing of the control pump in a 45° angle upwards. After all internal air in the pump and tubing has been expelled, tubing-shod mosquito hemostats should be applied a few centimeters from each end. Of note, only one ratchet click on each hemostat should be applied to maintain occlusive pressure without providing excessive force, which can damage the tubing. For antibiotic coated devices, the pump should be placed away from any other instruments and covered with a sterile drape until implantation.
The pressure regulating balloon should be prepared next. The balloon should be deflated manually. A 15 gauge blunt tip needle attached to a 30 mL syringe filled while 25 mL of fluid is inserted into the tubing then withdrawn to completely deflate the balloon. The balloon is then inflated with 20 mL of fluid. The balloon is rotated and the fluid and any air should be withdrawn to remove all possible air. A tubing-shod hemostat is then placed on the tubing again with only one click.
The cuff is the prepared in a similar fashion by attaching a blunt tipped 30 mL syringe, this time with 10 mL of fluid. The cuff should be completely deflated manually by withdrawing the syringe, then 3–4 mL of fluid (depending on the cuff size) should be instilled. Air bubbles should be manipulated manually out of the cuff while holding the syringe and tubing upright to allow them to escape into the superior aspect of the syringe. This can be repeated although overfilling the cuff should be avoided. After all air has been removed, apply two tubing-shod hemostats a few centimeters away from each other a few centimeters from the end of the tubing. The cuff should also then be set-aside in a dry, safe place under a sterile drape.
While device preparation is sometimes relegated to the surgical scrub nurse or other non-urologic personnel, it remains a critical step in urologic prosthetic surgery placement, and improper technique can lead to device malfunction or infection. This is especially true for smaller volume hospitals with rotating surgical teams, where an inexperienced individual may be the scrubbed assistant. Dedicated urologic nursing or ownership of the preparation portion of the procedure by the urologist can mitigate this effect. Critical steps include proper technique during removal of air bubbles from the multiple components of the device, which can prevent air locks and malfunctions. Generalized handling of the device is also extremely important to minimize complications. This includes appropriate clamping in terms of equipment (tubing-shod mosquito hemostats), positioning of the mosquito hemostats and use of a single ratchet click. Additionally, the device should be prepared and stored away from the rest of the operative tray, which may include sharp instruments that could damage the device. Finally, minimizing handling may limit the risk of inadvertent contamination, and this may be a strong argument towards having just one experienced urologist prepare and implant the device itself.
The AMS 800 can be filled with normal saline or radiographically opaque contrast. It is critical that if using contrast, sterile water (not saline) should be added in a specific amount to achieve an isotonic solution. Table 5.1 specifies the exact amount of contrast media and sterile water to mix. For example, when using Conray 43 (Mallinckrodt, Dublin, Ireland), 30 cc of contrast should be used with 60 cc of sterile H2O to create an appropriate dilution. The use of contrast in this setting is contraindicated in patients with iodine allergy.
Table 5.1
Recommended dilutions if using contrast for AUS filling (courtesy of AMS)
Contrast media | Dilution | Manufacturer | Validated for InhibiZone use | ||
|---|---|---|---|---|---|
Conray 43 | 30 cc Conray 43 | + | 60 cc sterile H2O | Mallinckrodt | Yes |
Cysto Conray II | 60 cc Cysto Conray II | + | 15 cc sterile H2O | Mallinckrodt | Yes |
Hypaque-Cysto | 60 cc Hypaque-Cysto | + | 58 cc sterile H2O | Nycomed | No |
Isovue 200 | 60 cc Isovue 200 | + | 23 cc sterile H2O | Bracco | No |
Isovue 300 | 57 cc Isovue 300 | + | 60 cc sterile H2O | Bracco | No |
Isovue 370 | 38 cc Isovue 370 | + | 60 cc sterile H2O | Bracco | No |
Omnipaque 180 | 60 cc Omnipaque 180 | + | 14 cc sterile H2O | Nycomed | No |
Omnipaque 240 | 60 cc Omnipaque 240 | + | 38 cc sterile H2O | Nycomed | No |
Omnipaque 300 | 57 cc Omnipaque 300 | + | 60 cc sterile H2O | Nycomed | Yes |
Omnipaque 350 | 48 cc Omnipaque 350 | + | 60 cc sterile H2O | Nycomed | No |
Telebrix 12 | 53 cc Telebrix 12 | + | 47 cc sterile H2O | Laboratoire Guerbel | Yes |
Cuff Sizing and Placement
After urethral dissection, the circumference of the urethra is measuring using the provided cuff sizer. Sizing should be performed around the corpus spongiosum with or without the catheter in place. While this is generally surgeon preference, one method should be used to maintain consistency of measurements for a single surgeon. Of note, the circumference of the inside of the sizer is slightly smaller than the outer circumference.
Determining the correct size of the cuff is critical to ensure appropriate coaptation of the urethra when the device is activated. Sizes vary from 3.5 to 11.0 cm in circumference. The 3.5 cm cuff has only been available since late 2009. The larger sizes are generally reserved for bladder neck placement. While many urologists favor implanting a cuff of the exact size that is measured, it is sometimes necessary or appropriate to choose a size slightly larger. One advantage of a larger size is to mitigate the risk of postoperative urinary retention, minimize the development of urethral atrophy or prevent risk of future erosion. The concern of placing a cuff too large is leaving the patient with ineffective urethral coaptation and poor continence results. However, there is some evidence that modest upsizing results in similar short-term continence and satisfaction while improving long term outcomes [59]. In the setting of urethral atrophy, a relatively standard 4.0–4.5 cm cuff may appear to be too large. Alternative operative techniques to deal with this clinical situation include placing a tandem cuff [36], urethral buttressing [60], or transcorporal placement [35]. Transcorporal placement involves additional dissection and will be discussed later. Especially in those patients with previous radiation, caution should be exercised when using a very tight cuff as radiation has been shown to significantly increase the risk of erosion when using the 3.5 cm cuff compared to larger sizes [61].
Once the desired size is determined, the cuff should be passed tab first under the urethra and the tubing should be passed through the tab hole to completely surround the urethra. Multiple shod hemostats are needed to successfully wrap the cuff around the urethra. Finally, the tab should be pulled over the tubing adapter (which appears as a button), which locks the cuff in position. Failure to adequately fasten the cuff can ultimately lead to failure if the cuff becomes unfastened. The small bit of tail remaining after fastening can be excised with blunt tipped scissors or left in place based on surgeon preference.
Reservoir Placement
Multiple alternative methods have been described for placement of the pressure regulating balloon such as scrotal or perineal, but a separate abdominal incision is generally recommended. After skin incision, Army Navy retractors are used to sweep away the superficial layers to reveal the anterior rectus or external oblique fascia. This is incised to allow separation of the muscle. Blunt finger dissection is usually sufficient to create adequate space in the space of Retzius for the balloon. When possible, preplacing fascial sutures prior to introducing the device can avoid inadvertent puncture. For prosthetic cases, absorbable monofilament suture may help to minimize infectious risks. When closing fascia, polydioxanone (PDS) suture is ideal given its longer time for absorption. For superficial layers including skin, faster absorbing suture is recommended.
A 61–70 cm H2O pressure regulating balloon is generally recommended for bulbar urethral cuff placement with 71–80 cm H2O reserved for cuffs at the bladder neck, which require a higher pressure to maintain closure. A deflated reservoir is inserted through the fascial defect, and the sutures are then tied around the tubing. Regardless of using normal saline or a diluted contrast, 22–24 mL of fluid is generally used to fill the pressure regulating balloon.
Pump Placement
Attention should next be turned to placement of the manual control pump. Through the incision for reservoir placement, blunt dissection is carried inferiorly into a dependent portion of the ipsilateral portion of the scrotum. It is important to avoid a superficial dissection (above Scarpa’s fascia), which can lead to palpable components and increase the risk of erosion. Great care should also be taken to avoid the testicular vessels. After the subdartos pouch is created, the pump is lowered into the space with the tubing visible from the incision. Figure 5.4 demonstrates this dissection.
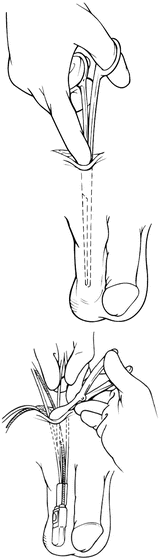

Fig. 5.4
Dissection for control pump (courtesy of AMS)
Connections
Connections are generally made through the abdominal incision. This includes connecting the tubing from the cuff to the pump (translucent clear) as well as the reservoir to the pump (clear with black stripes). The connections must be made carefully to avoid any pitfalls that could result in mechanical dysfunction. The AMS Quick Connect Sutureless Window Connectors have replaced the previous suture-tie connectors, which can still be used if necessary. The various shapes of connectors are shown in Fig. 5.5. The tubing should be cut using a straight scissors transversely to create a flat surface. It is important to instill saline into the components of the connector to avoid any air bubbles from being trapped internally. Additionally, multiple tubing-shod mosquito hemostats should be kept a few centimeters from the end of the tubing until the connection is completed. The collet rings are slipped over each end of the tubing with the teeth toward the middle, and the tubing is then inserted as far as possible until the central stop of the middle connector (Fig. 5.6). Through the two small windows, the tubing should be visible. At this point, the two collet rings should be seated into the middle connector and the squeeze tool should be applied in a fashion as to not trap any tubing material in the jaws. The squeeze tool is closed until the closure stops are flush with the central connector (Fig. 5.7). If using a straight connector, this is one application of the tool, but for right angle connections, the tool must be used twice (once for each side). After all the connections are complete, the hemostats should be removed, and the device should be tested for appropriate cycling.










