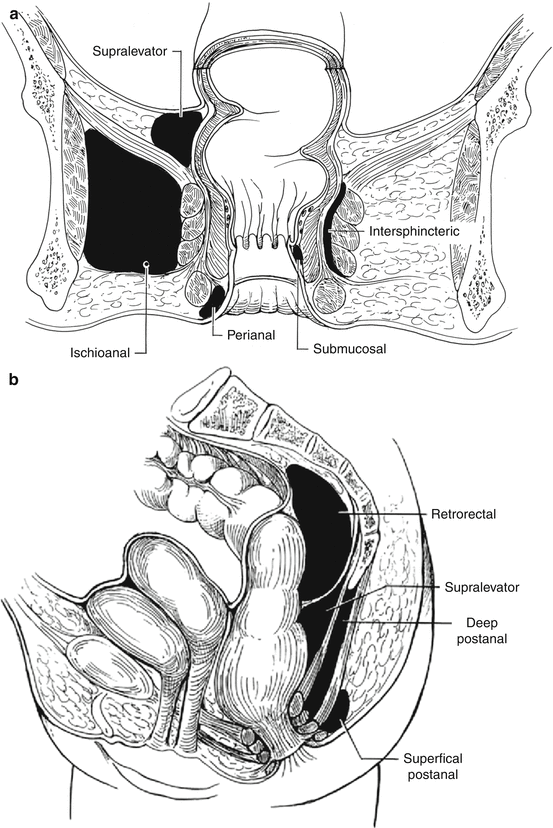
Potential space
Superior border
Anterior border
Inferior border
Posterior border
Lateral border
Medial border
Other features
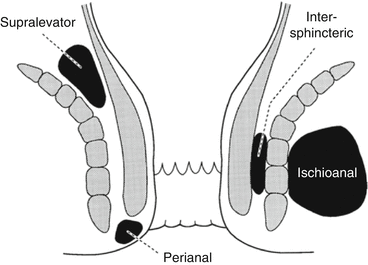
Perianal space
Anal verge
Becomes continuous with the ischioanal fat
Lower portion of anal canal
Continuous with the intersphincteric space and forms the most common abscess
Ischioanal space
Transverse perineal muscles
Lower border of the gluteus maximus and sacrotuberous ligament
Obturator internus
Levator ani and external sphincter muscle
Extends from the levator ani to the perineum. If the deep postanal space becomes infected, pus can spread circumferentially via the ischioanal space and this could form a horseshoe abscess
Intersphincteric space
Rectal wall
Continuous with perianal space
Lies between the internal and external sphincters. If infected, pus can spread
circumferentially
Supralevator space
Peritoneum
Levator ani muscle
Pelvic wall
Rectal wall
The rarest abscesses form from this space. If infected, pus can spread circumferentially
Deep postanal space
Levator ani muscle
Anococcygeal ligament
Tip of the coccyx
Pus can spread circumferentially via the ischioanal space and form a horseshoe abscess
Pathophysiology
Etiology
Table 13.2 lists the etiologies of anorectal abscesses. 90 % are from nonspecific cryptoglandular suppuration.
Table 13.2
Etiology of anorectal abscess
Nonspecific
Cryptoglandular
Specific
Inflammatory bowel disease
Crohn’s disease
Ulcerative colitis
Infection
Tuberculosis
Actinomycosis
Lymphogranuloma venereum
Trauma
Impalement
Foreign body
Surgery
Episiotomy
Hemorrhoidectomy
Prostatectomy
Malignancy
Carcinoma
Leukemia
Lymphoma
Radiation
Abscesses result from obstruction of the anal glands (Park’s cryptoglandular theory published in 1961).
Persistence of anal gland epithelium in the tract between the crypt and the blocked duct results in fistula formation.
Predisposing factors for abscess formation are diarrhea and trauma from hard stool.
Associated factors may be anal fissures, infection of a hematoma, or Crohn’s disease.
Classification
Evaluation
Symptoms
Anorectal pain, swelling, and fever.
Gluteal pain may accompany a supralevator abscess.
An intersphincteric or supralevator abscess may produce severe rectal pain with urinary symptoms (dysuria, retention, inability to void).
Physical Exam
On inspection, erythema, swelling, and possible fluctuation may be seen.
Digital exam may not be possible due to extreme pain.
Anoscopy and proctoscopy are avoided in the acute setting.
There may be no visible external manifestations despite severe rectal pain with an intersphincteric or supralevator abscess. If palpation is possible, a mass may be appreciated.
With a supralevator abscess, a tender mass may be palpated on rectal or vaginal exam.
Treatment
General Principles
The treatment of an anorectal abscess is prompt incision and drainage.
Watchful waiting with antibiotics is ineffective and may lead to a more complicated abscess with sphincter mechanism damage.
Delay in treatment may lead to a life-threatening necrotizing infection and death.
Operative Management
Incision and Drainage
A perianal abscess may be drained with local anesthesia. A cruciate or elliptical incision is made over the point of maximal tenderness and the edges trimmed to prevent premature closing (which could lead to recurrence). No packing is required.
Most ischioanal abscesses can be drained similarly to a perianal abscess, but the location of the incision should be shifted medial toward the anal side of the abscess but lateral to the external sphincter muscle (this minimizes the complexity if a fistula develops). Large abscesses or horseshoe abscesses (with the infection usually originating from the deep postanal space) often require drainage with regional or general anesthesia in the prone or left lateral position.
For a horseshoe abscess, a midline incision between the anus and coccyx is made and the superficial external sphincter muscle fibers are spread to enter the deep postanal space. Counter-incisions are made over each ischioanal fossa to allow drainage of the anterior extensions (Hanley procedure). The distal half of the internal sphincter may be divided to drain the gland where the infection originated (Fig. 13.3).
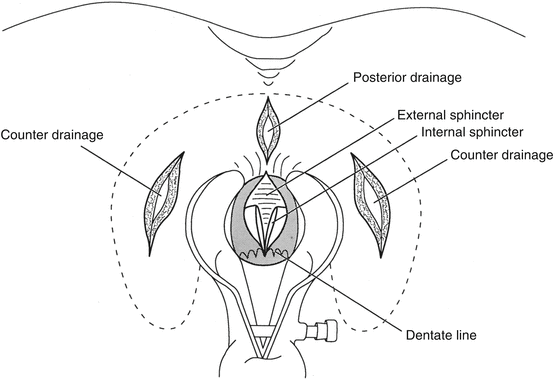
Fig. 13.3
Drainage of a horseshoe abscess
For pain out of proportion to physical findings, an exam under anesthesia is mandatory. An intersphincteric abscess may be established by palpation of a mass or aspiration of pus in the operating room. The treatment is division of the internal anal sphincter along the length of the abscess. The wound may be marsupialized for adequate drainage.
A supralevator abscess may result from an upward extension of an intersphincteric or ischioanal abscess or downward extension of a pelvic abscess. If the origin is from an intersphincteric abscess, drainage is accomplished through the rectum by dividing the internal sphincter (not through the ischioanal fossa as that would result in a suprasphincteric fistula). If the origin is an ischioanal abscess, this is drained through the perianal skin (not through the rectum as that would lead to an extrasphincteric fistula) (Fig. 13.4). If the abscess is of pelvic origin, it can be drained via the area it is pointing: through the rectum, ischioanal fossa, or percutaneously via the abdominal wall.
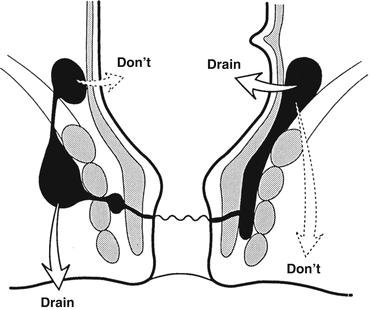
Fig. 13.4
Drainage of a supralevator abscess
Catheter Drainage
The area of maximal tenderness is prepped and the skin around it is infiltrated with local anesthesia (injecting at the maximal point of fluctuation may preclude the local anesthesia working in that acid environment).
A stab incision is made as close to the external sphincter muscle as possible so the tract is as short as possible in case a fistula develops.
A 10–16 French soft mushroom catheter is inserted over a probe into the cavity. It typically does not need to be sutured in place.
The catheter is shortened to 2–3 cm outside the skin with the tip in the depth of the abscess (Fig. 13.5a, b).
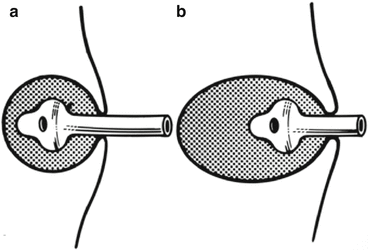
Fig. 13.5
Catheter in an abscess cavity: (a) The correct size and length of catheter. The size of the catheter should correspond to the size of the cavity. (b) When a catheter is too short. A catheter that is too short or too small could fall into the wound
The length of time that the catheter is left to drain the abscess cavity depends on the size of the abscess cavity, amount of granulation tissue around the catheter, and character and amount of drainage. If in doubt, it is better to leave it longer.
Primary Fistulotomy
Primary fistulotomy at the time of abscess drainage is controversial.
A meta-analysis showed that when the fistula is identified, drainage plus primary fistulotomy decreased the rate of subsequent fistula formation (by 83 %) with no increase in incontinence.
Against primary fistulotomy:
Difficulty in finding the internal opening (as high as 66 % of the time of abscess drainage) can lead to creation of a false passage and neglect to find the main source of infection.
Thirty-four to fifty percent of patients with first time abscess formation will not develop a fistula after drainage.
The search for an internal opening converts a procedure that can be done under local anesthesia (drainage) to one that requires regional or general anesthesia.
Those younger than 40 years old have a significantly higher risk of developing a fistula or recurrent abscess after initial drainage of a perianal abscess.
Abscess recurrence is more often observed after drainage of an ischioanal abscess.
If the internal opening of a low transsphincteric fistula is readily apparent at the time of abscess drainage, primary fistulotomy is feasible EXCEPT in patients with Crohn’s disease, acquired immune deficiency syndrome (AIDS), advanced age, high transsphincteric fistula, and an anterior fistula (in women).
Antibiotics
Antibiotics are only used as an adjunct for patients with valvular heart disease, prosthetic heart valves, extensive soft tissue cellulitis, prosthetic devices, diabetes, immunosuppression, or systemic sepsis.
Postoperative Care
Postoperatively, patients are instructed to take a regular diet, bulk-forming agents, the prescribed analgesia, and sitz baths.
Follow-up for patients is generally 2–4 weeks after the procedure, but those with an intersphincteric or supralevator abscess may be seen sooner at about 2 weeks.
If catheter drainage has been done, these patients are seen about 7–10 days after catheter placement. If the cavity has closed around the catheter and the drainage ceased, the catheter is removed. Otherwise, the catheter is left in place or a smaller catheter placed.
In all cases, patients are observed until complete healing occurs.
Complications
Recurrence
Up to 89 % of patients after drainage of an ischioanal or intersphincteric abscess will develop a recurrent abscess or fistula.
Recurrence is higher in those who had a previous abscess drained.
Recurrence of anorectal infections may be due to missed infections in adjacent anatomic spaces, presence of an undiagnosed fistula or abscess at the initial drainage, or failure to completely drain the initial abscess.
Extra-anal Causes
Extra-anal etiologies that can lead to abscess recurrence include hidradenitis suppurativa, pilonidal abscess (with downward extension), Crohn’s disease, tuberculosis, human immunodeficiency virus (HIV) infection, perianal actinomycosis, rectal duplication, lymphogranuloma venereum, trauma, foreign bodies, and perforated rectal carcinoma.
Incontinence
Iatrogenic injury can lead to incontinence, which occurs with division of external sphincter muscle during drainage of a perianal or deep postanal space abscess (in a patient with borderline continence) or division of puborectalis muscle in a patient with a supralevator abscess.
Prolonged packing of an abscess cavity may impair continence by leading to excessive scar formation.
Primary fistulotomy at the time of initial abscess drainage may lead to continence disturbances while unnecessarily dividing sphincter muscle.
Special Considerations
Necrotizing Anorectal Infections
Rarely, necrotizing anorectal infections may occur and could result in death.
Factors associated with this are delay in diagnosis and management, virulence of the organism, bacteremia, metastatic infections, underlying medical disorders (diabetes, blood dyscrasias, heart disease, chronic renal failure, hemorrhoids, previous abscess, or fistula), obesity, and cigarette smoking.
Symptoms and Signs
There are two types of presentation.
Group one: This group demonstrates superficial infection of the surrounding tissue including necrosis of the skin, subcutaneous tissue, fascia, and/or muscle. A black spot on the skin may occur early. Perianal crepitation, erythema, skin induration, blistering, or gangrene may be present.
Group two: This group presents with sepsis that involves the preperitoneal or retroperitoneal spaces. The signs may be subtle such as abdominal wall induration, tenderness, or a vague mass. Fever, tachycardia, and vascular volume depletion may precede appearance of an overt infection. CT scan is an excellent diagnostic tool (it will demonstrate origin and extent of infection).
Treatment
Early recognition, aggressive surgical debridement, and appropriate antibiotic administration are the most important factors to improve outcome and reduce mortality.
Vigorous resuscitation with invasive monitoring and respiratory and renal support is aggressively carried out (and treated).
Antibiotics effective against Staphylococci, Streptococci, gram-negative coliforms, Pseudomonas, Bacteroides, and Clostridium are administered intravenously. If a gram stain shows gram-positive rods, penicillin G (24–30 million units per day) and an aminoglycoside are given.
Tetanus toxoid is administered.
The goals of surgical debridement are to radically remove all nonviable tissue back to healthy tissue, halt infection progression, and alleviate systemic toxicity.
The skin changes may not reflect the severity of the liquefactive necrosis of the subcutaneous tissue and extensive necrosis of the underlying fascia. Reexamination under anesthesia is usually necessary to fully evaluate wounds for further debridement.
Vacuum-assisted closure may be helpful for healing these wounds, which may be quite extensive.
Colostomy is controversial, but should be considered if the sphincter muscle is grossly infected, the patient is incontinent, there is colonic or rectal perforation, or the patient is immunocompromised. A “medical colostomy” using enteral or parenteral nutrition has also been used.
Suprapubic urinary diversion is controversial and considered in the presence of a known urethral stricture or urinary extravasation with phlegmon.
Hyperbaric oxygen therapy (with 100 % oxygen via mask or endotracheal tube at 3ATM for 2 h over 1–2 treatments in patients without chronic obstructive pulmonary disease) has been advocated for patients with diffuse spreading infections and then also to promote wound healing. This does not eliminate the mandate for wide debridement of ischemic tissue.
High rates of mortality due to anorectal sepsis are related to extent of disease and the patient’s metabolic status at presentation. Mortality is two to three times higher in diabetics, the elderly, and patients with delayed treatment.
Anal Infection and Hematologic Diseases
There is a relationship between the number of circulating granulocytes and the incidence of perianal infections in patients with hematologic diseases (most occurred with fewer than 500 neutrophils per cubic millimeter).
The risk of developing anorectal infections is related to severity and duration of neutropenia.
The most important prognostic factor is the number of neutropenic days during the infectious episode.
Presenting symptoms commonly are fever preceding pain and urinary retention. Early signs are point anal tenderness and poorly demarcated induration. External swelling and fluctuation often appear late.
A neutropenic patient with perianal pain is assumed to have an acute anorectal infection and started on precautionary measures (no digital exams, suppositories, or enemas), sitz baths, stool softeners, bulk agents, analgesia, and antibiotics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree