Anejaculatory infertility is a challenge for the treating physician. Accurate diagnosis and choice of treatment plan must carefully proceed to maximize success rates while maintaining cost-effectiveness. In this article the authors review ejaculatory infertility evaluation and treatment.
Many normal physiologic functions need to come together to allow a man to father a child. The components of normal reproductive function include hormonal homeostasis, spermatogenesis, epididymal sperm transport and storage, normal erectile function, and finally, the ability to ejaculate intravaginally to deliver the sperm. Infertility may result from dysfunction on any of these levels.
Ejaculatory dysfunction is a relatively uncommon, but not rare, cause of infertility. Without adequate sperm delivery during intromission, the sperm and oocyte never meet. In this article we discuss the normal physiology of ejaculation and review the classification and causes of ejaculatory dysfunction. The evaluation of a patient who presents with possible ejaculatory dysfunction is presented, followed by a review of treatment modalities.
Anatomy of normal ejaculation
The ejaculatory reflex results from the interaction of anatomic structures that are under control of cerebral and peripheral neural pathways. The ejaculate is composed of fluids from various reproductive organs, which are presented here in order of decreasing volumetric contribution. The seminal vesicles supply approximately two thirds of the ejaculate volume and fructose and seminogellin, which contribute to semen coagulation. The prostate, which supplies approximately one third of the ejaculate volume from prostatic secretions, also secretes prostate-specific antigen, a proteolytic enzyme that cleaves seminogellin, effecting liquefaction . The testicular component, the sperm, comprises only approximately 1%–2% of the ejaculate volume. There is also a small contribution of fluid from the bulbourethral glands, and much of their output, a clear mucoid discharge, is released during sexual stimulation, before ejaculation. The bulbourethral glands are located in the pelvic floor striated muscle .
Fluids from the testicles and seminal vesicles enter the prostatic urethra via the ejaculatory ducts, which are formed from the coalescence of the ampulla of the vas deferens and the distal ducts of the seminal vesicles . The ejaculatory ducts course through the prostate as separate structures and enter the prostatic urethra lateral to the verumontanum. The arrangement of sphincter muscles in relation to the entry of the ejaculatory ducts is important. The internal sphincter/bladder neck is located proximal to the ejaculatory ducts, and because it contracts during seminal emission, it is able to prevent retrograde ejaculation into the bladder. The external sphincter, which is located distal to the ejaculatory duct openings, remains continent during emission and opens during ejaculation.
Normally during sexual stimulation, penile erection occurs to allow vaginal penetration. Erection is stimulated by parasympathetic fibers coursing to the corpus cavernosum. Erection is not a requirement for ejaculation but typically occurs before climax. In normal situations, erectile dysfunction naturally leads to infertility by preventing intromission. Erectile dysfunction–related infertility can be treated by obtaining a semen specimen by masturbation and using assisted reproductive techniques, such as artificial insemination.
Ejaculation itself results from coordination of various nervous system components. Cerebral input comes from erotic imagery and sensory and visual stimulation; cerebral influence in the ejaculatory process is poorly understood. Signals from upper centers travel down the thoracolumbar sympathetic nerves, resulting in the contraction of the prostatic smooth muscle and contraction of the seminal vesicles and vas deferens. The effector nerves that cause seminal emission and bladder neck closure are sympathetic fibers that arise from spinal levels T10-L2, coursing through the sympathetic ganglia, hypogastric plexus, and peripheral pelvic nerves . This bladder neck closure occurs simultaneously with the deposition of the seminal fluid in the posterior urethra.
Sensory input from penile stimulation is also important in the ejaculatory process. Penile stimulation courses through the dorsal nerves of the penis, paired in the dorsum of the penis glans and inserting into the pelvis, ultimately entering the spinal cord at S2-4. Efferents that arise from S2-4 travel via the pudendal nerves to pelvic floor and periurethral muscles. The well-known bulbocavernosal reflex travels this route. Stimulation of the glans results in a signal carried by dorsal nerves, enters the S2-4 spinal segment, and elicits a reflex that exits S2-4 and stimulates the pelvic floor skeletal muscle, which causes contraction.
Physiology of ejaculation
The events that occur during normal ejaculation include seminal emission, bladder neck contraction, and projectile ejaculation. During sexual stimulation, and most notably peripheral genital stimulation, there is involuntary tonic, low-level contraction of the periurethral/pelvic floor skeletal muscle. This contraction may be responsible for the “pre-ejaculate” that results from expulsion of bulbourethral gland secretions, due to muscular compression of the glands. With ongoing sexual stimulation there is persistent periurethral contraction, which eventually rises to high pressure within the prostatic urethra. The high pressure rise within the external sphincter rise seems to be a necessary prelude to the ejaculatory reflex .
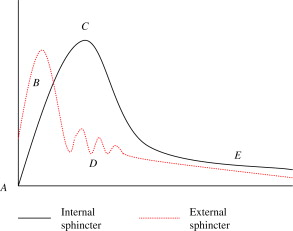
After a rather rapid rise to peak contraction pressures of the external sphincter, internal sphincter pressure rises rapidly over a few seconds. It is possible the sensation of genital orgasm results from the high pressure contraction of these sphincter muscles. When the internal sphincter pressure rises, external sphincter pressure drops, and at the same time the ejaculatory organs contract, expelling their contents into the urethra, which is the process of emission. With the internal sphincter tightly closed in tonic contraction, a sequence of involuntary rhythmic contractions of the periuretheral muscles occurs, leading to a pulsatile, projectile ejaculation phase. The internal sphincter remains tightly closed during this time to prevent retrograde ejaculation . The rhythmic contractions were previously thought to be a reflex response to distension of the posterior urethra during emission, but this supposition does not seem to be the case, because these contractions take place as part of the ejaculatory reflex, even in medical conditions in which seminal emission is absent. A timeline showing the sequence of events during ejaculation is found in Fig. 1 .
Sperm are transported from the storage site in the cauda epididymis in response to sexual stimulation and proceed rapidly through the vas deferens during seminal emission. After ejaculation, vasal sperm are transported back to the cauda epididymis, where they are stored in a friendly environment . Contrary to common thought, sperm are not stored in the seminal vesicles, which is a hostile environment. In certain clinical conditions, such as ejaculatory duct partial obstruction , prolonged abstinence, and spinal cord injury (SCI) , sperm may be stored in the seminal vesicles, and poor sperm motility results.
Physiology of ejaculation
The events that occur during normal ejaculation include seminal emission, bladder neck contraction, and projectile ejaculation. During sexual stimulation, and most notably peripheral genital stimulation, there is involuntary tonic, low-level contraction of the periurethral/pelvic floor skeletal muscle. This contraction may be responsible for the “pre-ejaculate” that results from expulsion of bulbourethral gland secretions, due to muscular compression of the glands. With ongoing sexual stimulation there is persistent periurethral contraction, which eventually rises to high pressure within the prostatic urethra. The high pressure rise within the external sphincter rise seems to be a necessary prelude to the ejaculatory reflex .
After a rather rapid rise to peak contraction pressures of the external sphincter, internal sphincter pressure rises rapidly over a few seconds. It is possible the sensation of genital orgasm results from the high pressure contraction of these sphincter muscles. When the internal sphincter pressure rises, external sphincter pressure drops, and at the same time the ejaculatory organs contract, expelling their contents into the urethra, which is the process of emission. With the internal sphincter tightly closed in tonic contraction, a sequence of involuntary rhythmic contractions of the periuretheral muscles occurs, leading to a pulsatile, projectile ejaculation phase. The internal sphincter remains tightly closed during this time to prevent retrograde ejaculation . The rhythmic contractions were previously thought to be a reflex response to distension of the posterior urethra during emission, but this supposition does not seem to be the case, because these contractions take place as part of the ejaculatory reflex, even in medical conditions in which seminal emission is absent. A timeline showing the sequence of events during ejaculation is found in Fig. 1 .
Sperm are transported from the storage site in the cauda epididymis in response to sexual stimulation and proceed rapidly through the vas deferens during seminal emission. After ejaculation, vasal sperm are transported back to the cauda epididymis, where they are stored in a friendly environment . Contrary to common thought, sperm are not stored in the seminal vesicles, which is a hostile environment. In certain clinical conditions, such as ejaculatory duct partial obstruction , prolonged abstinence, and spinal cord injury (SCI) , sperm may be stored in the seminal vesicles, and poor sperm motility results.
Types of ejaculatory dysfunction
There are several broad categories of ejaculatory dysfunction, which are listed in Box 1 .
Premature ejaculation
Neurogenic anejaculation
Retrograde ejaculation
Iatrogenic causes
Idiopathic anejaculation/anorgasmia
Aperistalsis of the vas deferens
In this issue of the Urologic Clinics , the topic of ejaculatory duct obstruction is covered in a separate article and is not discussed further here. Idiopathic aperistalsis of the vas deferens has been described. It is treated with sympathomimetic agents but remains poorly characterized and also is not discussed further here.
Premature ejaculation (PE) may be the most common male sexual dysfunction and has been reported to affect up to 31% of men aged 18 to 59 . It leads to significant distress in many couples. PE may be lifelong or acquired. It is thought that men with lifelong PE likely suffer from a physiologic difference in the ejaculatory threshold when compared with normal men and may need medical therapies. Men with acquired PE are better candidates for cognitive-behavioral therapy . Medical therapy in the past has consisted of chronic selective serotonin reuptake inhibitors, and on-demand selective serotonin reuptake inhibitors. A rapid-onset, short-acting selective serotonin reuptake inhibitor, dapoxetine, has undergone US Food and Drug Administration review but has not been approved to date .
Prolonging the intravaginal ejaculatory latency time gives significant relief and improvement of the sexual experience. Because ejaculation in patients who have PE usually occurs intravaginally, however, it rarely causes infertility. If ejaculation occurs before intromission, however, couples can perform home-based intravaginal insemination to circumvent the fertility problem. Because PE does not usually lead to infertility, it is not discussed further in this article.
Neurogenic anejaculation
SCI is the most common cause of neurogenic anejaculation. Men who have SCI suffer from erectile and ejaculatory dysfunction. Many men with SCI, especially those with upper motor neuron lesions, have reflex erections and some degree of ability to engage in vaginal intercourse. Even men with short-lived reflex erections usually respond to oral erectogenic agents or penile injection of vasoactive agents. The greater problem that leads to infertility in men with SCI is absence of ejaculation, which affects most men with spinal lesions .
A frequent component of the treatment plan for testicular cancer is a retroperitoneal lymph node dissection (RPLND). Although this treatment has contributed to the current excellent survival from this previously lethal disease, we currently have good fortune of treating infertility in the survivors. The surgical field in the classical operation removes the postganglionic sympathetic nerves exiting the sympathetic chains and the hypogastric plexus, effectively removing the efferent stimulation for seminal emission and bladder neck closure .
Nerve-sparing RPLND procedures have been devised to preserve antegrade ejaculation . Crucial areas to preserve during such procedures include the hypogastric plexus anterior to the aorta below the inferior mesenteric artery and the postganglionic sympathetic nerves from T10-L2. Templates designed to unilaterally preserve at least one side are successful, but when combined with meticulous dissection of bilateral sympathetic nerves, the preservation rate of antegrade ejaculation approaches 100%. Some situations still exist in which surgery results in ejaculatory dysfunction. Large tumor burden or RPLND surgeries performed after chemotherapy give high risk for ejaculatory dysfunction. The risk of surgical sympathectomy is not limited to RPLND. Any surgery that is performed in the periaortic or pelvic regions can effect ejaculation, including aortic aneurysm surgery and aortic bypass surgeries . Any retroperitoneal lymph node samplings or trauma surgeries also can result in problems.
Patients with post-RPLND ejaculatory dysfunction present with a normal feeling of orgasm and ejaculation. This presentation leads many clinicians to refer to post-RPLND ejaculatory dysfunction as “retrograde ejaculation.” although this is not the case. A study by Kedia and colleagues suggested that if a dry orgasm is noted after RPLND, there is a high likelihood that this symptom represents total absence of ejaculation and not simply a retrograde ejaculation situation. These patients experience a failure of emission (see previous discussion) and thus experience a “dry ejaculate.”
Although it is commonly recognized that men with diabetes mellitus are at risk for complications of retinopathy, vasculopathy, and neuropathy, the effect of diabetes on sexual function is less well known. Erectile dysfunction is the most common sexual issue related to diabetes, but ejaculatory problems are also common . Control of blood sugar (or lack thereof) is directly related to the risk of complications . Men with ejaculation changes caused by diabetes exhibit a slowly progressive decline in ejaculatory function . Typically, the first symptom is a decrease in the amount of ejaculate, which progresses to retrograde ejaculation, with postclimax urine cloudiness, to loss of the cloudiness, which is consistent with loss of emission.
Congenital spinal anomalies, such as spina bifida, also can impair ejaculation. Occasionally men may present with lifelong or acquired anejaculation and can be found to have an occult dysplasia of the lower spinal cord, possibly with a tethered cord syndrome. Men with lifelong problems may have poorer sperm quality than men with acquired SCI. Other neurologic conditions that affect spinal cord function or its sympathetic outflow can result in ejaculatory dysfunction; examples include multiple sclerosis, transverse myelitis, and vascular spine injuries. These disorders resemble the SCI group in their dysfunction.
Retrograde ejaculation
In retrograde ejaculation, all the components of the ejaculatory reflex are present, except for bladder neck closure. As discussed earlier, bladder neck closure occurs because of high pressure. If this closure cannot occur because of anatomic reasons (eg, after prostate resection) or physiologic reasons (eg, diabetes mellitus), the result is retrograde ejaculation. In the absence of adequate internal sphincter contraction, the emitted semen takes the path of least resistance and flows backward into the bladder. The patient might notice that the postorgasm urine is cloudy because of the presence of semen.
Because seminal emission and bladder neck closure are both controlled by alpha-adrenergic neurons, all of the causes listed for neurogenic anejaculation may cause retrograde ejaculation instead of absent emission. It is a matter of the degree of the neural dysfunction. Some other causes warrant mention, however, because they more commonly cause retrograde ejaculation, with little or no diminution of the amount of emission.
Iatrogenic causes of ejaculation dysfunction
Although not specifically neurogenic, medications can prevent climax and therefore ejaculation. Selective serotonin reuptake inhibitors and tricyclic antidepressants are the most common. Because these drugs are used to treat PE, administration to a normal individual can result in dysfunction. When all types of antidepressants are considered, the rate of male sexual dysfunction with antidepressants is as high as 62.4% . Certain medications usually do not limit the ability to achieve climax and seminal emission but can specifically impair bladder neck contraction. The most common class is the alpha-adrenergic antagonists. These drugs may be either nonselective, such as terazosin, which is given for systemic hypertension, or genitourinary selective, such as tamsulosin or alfuzosin, which are for prostatic obstruction. Usually there is some diminution in seminal emission, but most of these individuals who have dysfunction from alpha blockers have retrograde ejaculation .
Anatomic opening of the bladder neck is usually postsurgical. In the past, Y-V plasty of the bladder neck was a common treatment for recurrent urinary infections in boys, but that has fallen out of favor. It remains possible, however, that a patient with a history of this archaic procedure still will present to an infertility setting. Currently, transurethral prostatectomy or prostatic ablative procedures are the most common cause of anatomic retrograde ejaculation .
Idiopathic anejaculation/anorgasmia
The word “idiopathic” is used frequently when we cannot determine the accurate physiologic cause of a problem, but it is also used frequently when describing conditions that are functional. With idiopathic anejaculation, the latter use of the word seems appropriate. Although there may be some undiagnosed neuropathic/physical problem in these patients, most practitioners in the field believe that idiopathic anejaculation/anorgasmia is a psychogenic problem .
The characteristics of the condition support this conclusion. There are no demonstrable neurologic derangements. Several reports link inability to achieve orgasm in otherwise healthy individuals to other psychogenic issues, such as anxiety. Men with this condition tend to have a history of religiously strict upbringing . The history of these men is often revealing. Some are able to climax and ejaculate with masturbation but not with intercourse. More often, however, there has never been a climax while awake. Notably, most men with this condition have intermittent nocturnal emissions , and some awaken during the event. The fact that ejaculation occurs during sleep suggests that this condition is psychogenic, much the same as nocturnal erectile function supports a psychogenic source of erectile dysfunction while awake.
Recently, Perelman described a subset of men with acquired inability to climax during sex that is related to frequent masturbation or use of idiosyncratic masturbation techniques. He believes that these men are conditioned to respond to the masturbatory technique and not to the sensation of vaginal intercourse. Changing the stimulation technique and decreasing the frequency of masturbation have been successful in reversing the problem . It is reasonable to treat men with idiopathic anejaculation with sex therapy techniques. Success rates other than the subset reported by Perelman, however, are poor. Men with refractory inability to ejaculate, despite a course of sex therapy, may be candidates for ejaculation induction procedures . For men interested in fertility, electroejaculation (EEJ) or surgical sperm retrieval techniques can be used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





