CHAPTER 47 Anatomy, Histology, Embryology, and Developmental Anomalies of the Stomach and Duodenum
EMBRYOLOGY AND ANATOMY OF THE STOMACH
GENERAL CONSIDERATIONS
The stomach is recognizable in the fourth week of gestation as a dilation of the distal foregut (Fig. 47-1).1 As the stomach enlarges, the dorsal aspect grows more rapidly than the ventral aspect, thus forming the greater curvature. Additionally, during the enlargement process the stomach rotates 90 degrees around its longitudinal axis, orienting the greater curvature (the dorsal aspect) to the left and the lesser curvature (ventral aspect) to the right. The combined effects of rotation and ongoing differential growth result in the stomach lying transversely in the mid and left upper abdomen. The events also explain the vagal innervation of the stomach: the right vagus nerve innervating the posterior stomach wall (the primordial right side) and the left vagus nerve innervating the anterior wall (the primordial left side).
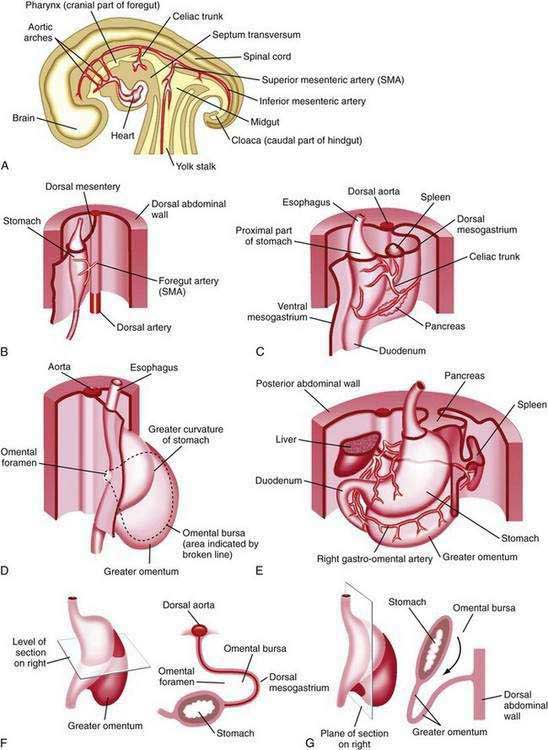
Figure 47-1. Development of the stomach and duodenum and formation of the omental bursa (lesser sac) and greater omentum. A, Median section of a 28-day embryo. B, Anterolateral view of a 28-day embryo. C, Embryo about 35 days old. D, Embryo about 40 days old. E, Embryo about 48 days old. F, Lateral view of the stomach and greater omentum of an embryo at about 52 days. The transverse section shows the omental foramen and omental bursa. G, Sagittal section showing the omental bursa and greater omentum. The embryology of the duodenum is discussed further in Chapters 55 and 96.
(From Moore KL, Persaud TVN. The developing human. 7th ed. Philadelphia: WB Saunders; 2003. p 258.)
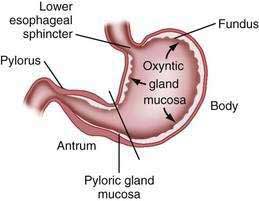
The stomach is divided into four regions that can be defined by anatomic or histologic landmarks (Fig. 47-2).2 Anatomically the cardia is a small ill-defined area of the stomach immediately adjacent to its junction with the esophagus. This region of the stomach has been the focus of intense investigation. Controversy exists as to the nature, location, extent, and even existence of cardiac mucosa. The fundus projects upward, above the cardia and gastroesophageal junction. This dome-shaped area of the stomach is its most superior portion and is in contact above with the left hemidiaphragm and to the left with the spleen. The body, or corpus, the largest portion of the stomach, is located immediately below and continuous with the fundus. The incisura angularis, a fixed, sharp indentation two thirds of the distance down the lesser curvature, marks the caudal aspect of the gastric body (Fig. 47-3). The gastric antrum extends from its indistinct border with the body to the junction of the pylorus with the duodenum. These gross anatomic landmarks correspond roughly with the mucosal histology because antral mucosa (pyloric gland mucosa) actually extends from an area on the lesser curvature somewhat above the incisura. The pylorus (pyloric channel) is a tubular structure joining the duodenum to the stomach and contains the palpable circular muscle, the pyloric sphincter. The pylorus is somewhat mobile owing to its enclosure between the peritoneum of the greater and lesser omenta but is generally located 2 cm to the right of midline at L1. Corresponding motor and secretory functions of these regions of the stomach are discussed in detail in Chapters 48 and 49.
TISSUE LAYERS OF THE STOMACH
The luminal surface of the gastric wall forms thick, longitudinally oriented folds, or rugae, that flatten with distention. Four layers make up the gastric wall: mucosa, submucosa, muscularis propria, and serosa. Mucosa lines the gastric lumen, appearing as a smooth, velvety blood-filled lining. The mucosa of the cardia, antrum, and pylorus is somewhat paler than that of the fundus and body. It is within the gastric mucosa that most of the functional secretory elements of the stomach are located (see Chapter 49). The submucosa, immediately deep to the mucosa, provides the dense connective tissue skeleton of collagen and elastin fibers. Lymphocytes, plasma cells, arterioles, venules, lymphatics, and the submucosal plexus are also contained within the submucosa. The third tissue layer, the muscularis propria, is a combination of three muscle layers: inner oblique, middle circular, and outer longitudinal. The inner oblique muscle fibers course over the gastric fundus, covering the anterior and posterior aspects of the stomach wall. The middle circular fibers encircle the body of the stomach, thickening distally to become the pyloric sphincter. The outer longitudinal muscle fibers course primarily along the greater and lesser curvatures of the stomach. The final layer of the stomach is the transparent serosa, a continuation of the visceral peritoneum.
MICROSCOPIC ANATOMY
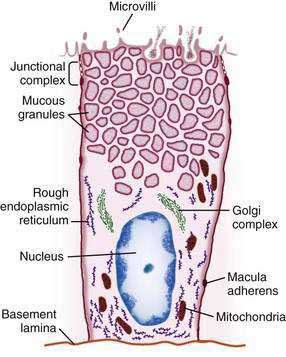
The gastric mucosal surface is composed primarily of a simple layer of columnar epithelial cells 20 to 40 mm in height. These surface mucous cells (Fig. 47-4), which are similar throughout the stomach, contain basally located nuclei, prominent Golgi stacks, and dense cytoplasm with especially apically dense mucin-containing membrane-bound granules. The cells secrete mucus in granules that are released via exocytosis, apical expulsion, and cell exfoliation. The primary role of mucus, along with bicarbonate, is luminal cytoprotection from “the elements”: acid, pepsin, ingested substances, and pathogens. Cellular renewal time for a gastric surface mucous cell is approximately three days.
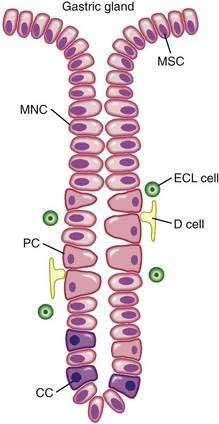
The surface epithelial lining is invaginated by gastric pits, or foveolae, that provide the gastric glands access to the gastric lumen, with a ratio of one pit to four or five gastric glands. The gastric glands of different anatomic regions of the stomach are lined with different types of specialized epithelial cells, allowing for differentiation of these regions by type of gastric gland (see Fig. 47-2). The first region, the cardia, is a small transition zone from esophageal squamous epithelium to gastric columnar epithelium. The cardia has been a controversial histologic area of discussion, with theories suggesting that its presence is pathologic. However, recent observations concluded that cardiac mucosa develops during gestation and is present at birth.3 The cardiac glands have a branched and tortuous configuration and are populated by mucous, endocrine, and undifferentiated cells. There is a gradual transition from cardiac glands to the second region, the acid-secreting segment of the stomach. This region encompasses the gastric fundus and body and contains the parietal (or oxyntic or fundic) glands. Parietal, chief (also known as peptic), endocrine, mucous neck, and undifferentiated cells compose the oxyntic glands. The final region, corresponding to the antrum and pylorus, contains the pyloric glands, composed of endocrine cells, including gastrin-producing G cells and mucous cells.
By far the most numerous and distinctive gastric glands are the oxyntic glands (Fig. 47-5), responsible for the secretion of acid, intrinsic factor, and most gastric enzymes. These fairly straight and simple tubular glands are closely associated in the areas of gastric fundus and body. A typical gland is subdivided into three areas: the isthmus (where surface mucous cells predominate), the neck (where parietal and mucous neck cells predominate), and the base (where chief cells predominate, along with some parietal and mucous neck cells). Endocrine cells, somatostatin-containing D cells, and histamine-secreting enterochromaffin-like (ECL) cells are scattered throughout the oxyntic epithelium.
The principal cell type of the oxyntic gland is the parietal cell (Fig. 47-6), responsible for the oxyntic mucosal secretion of 3 × 106 hydrogen ions per second, at a final hydrochloric acid (HCl) concentration of around 150 mmol/L. Parietal cells bulge into the lumina of the oxyntic glands and, as the primary hydrogen secretors, have ultrastructural characteristics different from other gastric cells: large mitochondria, microvilli lacking in glycocalyx, and a cytoplasmic canaliculi system in contact with the lumen. In the nonsecreting parietal cell, a cytoplasmic tubulovesicular system predominates and short microvilli line the apical canaliculus. In the secreting state, the tubulovesicular system disappears, leaving an extensive system of intracellular canaliculi containing long microvilli. Mitochondria occupy approximately 30% to 40% of the secreting parietal cell volume, providing energy required for acid secretion across apical microvilli (see Fig. 47-6). The so-called proton pump—the H+,K+-ATPase—resides in the apical microvillus membrane, as does carbonic anhydrase. The apical H+,K+-ATPase functions as the proton translocator in gastric acid secretion (see Chapter 49). Acid secretion begins within 5 to 10 minutes of stimulation. Additionally, parietal cells are the site of intrinsic factor secretion via membrane-associated vesicle transport.
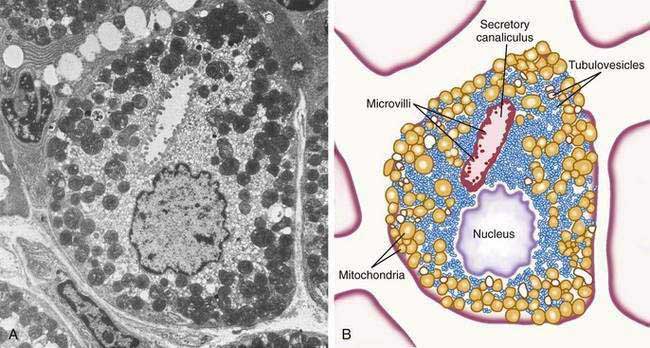
Figure 47-6. Parietal cell. A, Electron photomicrograph. B, Schematic.
(A and B, from Johnson LR. Gastrointestinal physiology. 6th ed. St Louis: Mosby; 2001. pp 78, 79.)
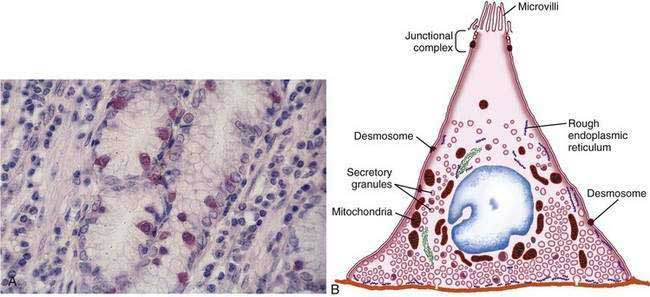
The final region of the stomach encompasses the antrum and pylorus and contains extensively coiled antral glands composed of endocrine and epithelial cells. The epithelial cells are predominantly mucous cells, and there are small numbers of pepsinogen II–secreting oxyntic cells. Although also small in number, gastrin-secreting (G) cells play a vital physiologic role and are the prototype of the open enteroendocrine cell. These cells, which occur either singly or in small clusters in the mid- to deep sections of antral glands (Fig. 47-7A), contain a basilar cytoplasm densely packed with gastrin-containing secretory granules (see Fig. 47-7B). Gastrin release is stimulated by gastric distention, vagal stimulation, dietary amino acids, and peptide, with rapid appearance of the hormone into the bloodstream in the postprandial period (see Chapter 49). The apical or luminal surface of the G cell is narrowed into small microvilli thought to contain receptors responsible for amino acid and peptide stimulation of gastrin release. Significant quantities of gastrin are also secreted into the gastric lumen; gastrin is a known gastric growth and differentiation factor, mediated through upregulation of heparin-binding epidermal-like growth factor (HB-EGF) in gastric parietal cells.4,5
Antral enteroendocrine D cells found in close association with G cells manufacture somatostatin, a potent inhibitor of gastrin secretion. The D cells are also present in small numbers in oxyntic glands. Somatostatin is thought to inhibit acid secretion through paracrine (direct action on ECL and perhaps parietal cells or indirect action on G cells) or endocrine effects (direct action on parietal cells) (see Chapter 49 for more details).