CHAPTER 55 Anatomy, Histology, Embryology, and Developmental Anomalies of the Pancreas
The pancreas was one of the last organs in the abdomen to receive the critical attention of anatomists, physiologists, physicians, and surgeons.1,2 It was first referred to as the “finger of the liver” in the Talmud, written between 200 bc and ad 200. Galen named it (though Ruphos, circa ad 100, should probably be credited2), and thought the pancreas served to support and protect blood vessels. Vesalius considered the organ a cushion for the stomach. Little further information was available until Wirsung demonstrated the pancreatic ducts of humans in 1642 and de Graaf discovered pancreatic secretion from the pancreatic fistula of dogs in 1664.
The histologic structure of the pancreas was first described in 1869 by Langerhans. Shortly thereafter, Heidenhain3 characterized the periodic postprandial changes that occurred in the histology of the canine pancreas. He found that as the granular regions of cells disappeared after feeding, the enzyme activity in pancreatic juice increased; he concluded that the granules contained the precursors of the digestive enzymes.
ANATOMY
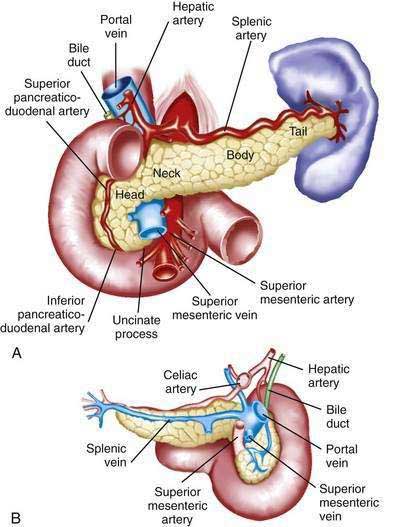
The pancreas is a soft, elongated, flattened gland 12 to 20 cm in length.4–6 The adult gland weighs between 70 and 110 g. The head lies behind the peritoneum of the posterior abdominal wall and has a lobular structure. The pancreas is covered with a fine connective tissue but does not have a true capsule. The head of the pancreas is on the right side and lies within the curvature of the duodenum. The neck, body, and tail of the pancreas lie obliquely in the posterior abdomen, with the tail extending as far as the gastric surface of the spleen (Fig. 55-1).
The body passes laterally and merges with the tail of the pancreas without a discernible junction point. The tail is relatively mobile, its tip usually reaching the hilus of the spleen. With the splenic artery and vein, the tail is contained between the two layers of the splenorenal ligament. The splenocolic ligament attaches the splenic flexure of the colon to the spleen and brings it near the tail of the pancreas. The relationship of the pancreas to important structures in the posterior abdomen is seen in Figure 55-2.
The distal end of the common bile duct, the duodenum, and the head of the pancreas form a unit. The common bile duct is located to the right of the gastroduodenal artery in the posterior wall of the duodenum. The bile duct passes through the substance of the pancreatic head, usually to join with the main pancreatic duct for some distance to reach the duodenal papilla (Fig. 55-3A).
DUCTAL STRUCTURES
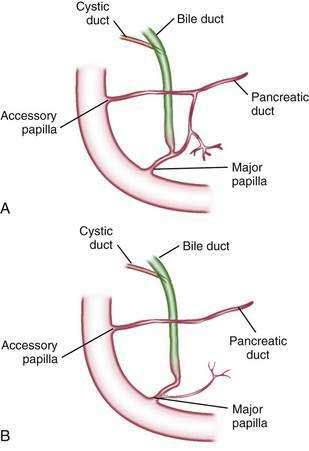
The main pancreatic duct (of Wirsung) begins near the tail of the pancreas. It is formed from anastomosing ductules draining the lobules of the gland. It courses left to right and is enlarged by additional ducts. Through the tail and body, the duct lies midway between the superior and inferior margins and slightly posterior. The main duct turns caudal and posterior on reaching the head of the pancreas. At the level of the major papilla, the duct turns horizontally to join usually with the common bile duct (see Fig. 55-3A). This short common segment is the ampulla of the bile duct, which terminates in the duodenal papilla.
The relationship of the common bile duct and the duct of Wirsung at the papilla is complex. The ducts may open separately at the ampulla and have an interposed septum or may have a common channel. A common channel for bile and pancreatic secretion is ordinarily formed by the absence of a septum between the biliary and pancreatic ducts as they approach the ampulla of Vater. In adults studied by endoscopic retrograde cholangiopancreatography (ERCP), the length of the common channel averages 4.5 mm, with a range of 1 to 12 mm.7,8 In various series, more than two thirds of patients had some degree of a common channel.9–13 In a large autopsy series, 74% of patients had a common channel, 19% had separate openings, and 7% had an interposed septum.9
The accessory pancreatic duct of Santorini is frequently present and usually communicates with the main duct (see Fig. 55-3A). The accessory duct lies anterior to the bile duct and usually drains into the minor papilla, which lies proximal to the ampulla of Vater in the second duodenum. The accessory duct is patent in 70% of autopsy specimens. In about 10% of individuals there is no connection between the accessory duct and the main duct.14 A number of variations in the two pancreatic ducts may be encountered.
The greatest diameter of the main pancreatic duct is in the head of the pancreas, and the duct gradually tapers, progressing to the tail of the pancreas. The main duct ranges from 3.1 to 4.8 mm in the head of the pancreas and tapers to 0.9 to 2.4 mm in the tail.15 Specific normal limits of pancreatic duct diameter in the head (4 to 5 mm), body (3 to 4 mm), and tail (2 to 3 mm) are generally accepted. However, studies have shown an increase in pancreatic duct size with age and pancreatic disease.16–18
CIRCULATION
The pancreas has a rich circulation derived from branches of the celiac and superior mesenteric arteries.18,19 The head of the pancreas and surrounding duodenum are supplied by two pancreaticoduodenal arterial arcades. They are formed by the anterior and posterior superior pancreaticoduodenal arteries from the hepatic branch of the celiac artery that join a second pair of anterior and posterior inferior pancreaticoduodenal arteries.
LYMPHATIC DRAINAGE
The lymphatics, in general, drain the surface network of lymph toward regional nodes and are formed near the larger blood vessels.20,21 The superior lymphatic vessels run along the upper border of the pancreas closely with the splenic blood vessels. Those on the left side of the body and tail empty into nodes in the splenic hilum. Those on the right side of the body and the pancreatic neck empty into nodes near the upper border of the head. They also receive tributaries from the anterior and posterior pancreatic surfaces. The inferior lymphatic vessels run with the inferior pancreatic artery. Those that drain the lower left side of the body and tail drain toward nodes in the splenic hilum. The remaining regions of the neck and body drain toward the right.
HISTOLOGY AND ULTRASTRUCTURE
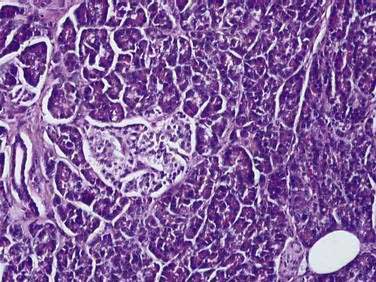
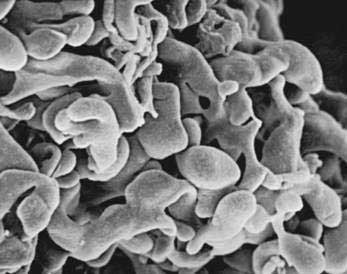
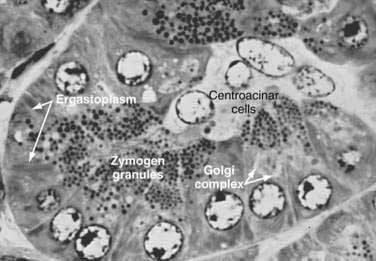
The pancreas is a compound, finely nodular gland that is grossly similar to but less compact than the salivary glands. It is surrounded by fine connective tissue but does not have a fibrous tissue capsule. The lobules are visible on gross examination and are connected by connective tissue septa that contain the blood vessels, nerves, lymphatics, and excretory ducts (constituting about 18% of this organ). The gland is a mixed exocrine (about 80%) and endocrine (about 2%) organ (Fig. 55-4). The endocrine portion consists of the islets of Langerhans, which are spherical clusters of light-staining cells scattered throughout the pancreas (see Chapter 1). The exocrine portion consists of numerous dark-staining acini composed of tubular and spherical masses of cells, which are the subunits of the lobule.22,23 Silicone casts of the duct lumen formed by retrograde injection indicate that the tubular portions of the acini are extensive and that the exocrine cells are arranged primarily as curved, branching tubules that anastomose and end blindly (Fig. 55-5).24
The lumen of the acinus is the origin of the secretory duct and contains centroacinar cells, which are unique to the pancreas. These cells are pale staining in histologic sections and smaller than the acinar cells. The lumen of the acinus leads into the intralobular ducts, which are covered by low columnar epithelial cells similar in appearance to the centroacinar cells. These ducts are nonstriated and anastomose to form the interlobular ducts, which are lined by a columnar epithelium (see Fig. 55-4). Goblet cells and occasional argentaffin cells also are present. The interlobular ducts anastomose to become the main pancreatic duct. The larger ducts have a somewhat thick wall consisting of connective tissue and elastic fibers. Acinar, ductal, and islet cells can be distinguished by monoclonal antibodies specifically reactive with these cell types.25–27
Acinar cells are tall, pyramidal, or columnar epithelial cells, with their broad bases on a basal lamina and their apices converging on a central lumen (Fig. 55-6). In the resting state, numerous eosinophilic zymogen granules fill the apical portion of the cell. The basal portion of the cells contains one or two centrally located, spherical nuclei and extremely basophilic cytoplasm. The Golgi complex lies between the nucleus and zymogen granules and can be seen as a clear, nonstaining region (see Fig. 55-6).
The acinar cells undergo cyclic changes in morphology in response to feeding and digestion.28,29 After a large meal, the zymogen granule content of the cells is depleted. This apparently occurs by a decrease in the size and number of granules.28 After depletion of the granules, the Golgi apparatus may be observed at the apex of the cell and appears more extensive than in the resting state. The reductions in size and number of granules occur with a substantial increase in pancreatic enzyme secretion (see Chapter 56).
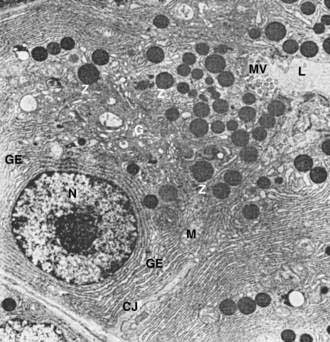
The subcellular structure of the acinar cells can be visualized at the electron-microscopic level (Fig. 55-7). The acinar cell has several short, slender microvilli about 0.2 µm in length that extend into the lumen of the acinus. The lumen typically contains flocculent electron-dense material, which presumably is the secreted digestive enzymes. Thin filaments form the axis of the microvilli as well as a network beneath the apical plasmalemma. These microfilaments apparently play a structural role because their disruption causes expansion of the acinar lumen and loss of microvilli.30 Adjacent cells are joined at the apical surface by electron-dense intercellular junctions. Tight junctions form a belt-like band around the apical end of the cell and are produced by the apposition of the external membrane leaflets of neighboring cells.31 These junctions prevent the reflux of secreted substances from the duct into the intercellular space. Gap junctions are distributed on the lateral cellular membranes and are formed by the apposition of larger, disk-shaped membrane plaques. They allow communication between cells. Below the junctions, the lateral cell borders are relatively straight and have a few, small interdigitations. Pancreatic lateral cell membranes display unique antigenic determinants that are not found on apical cell membranes.32
The nucleus usually is spherical, about 6 µm in diameter,33 with one or more nucleoli in the interior and patches of dense heterochromatin along the inner nuclear membrane. Numerous conspicuous nuclear pores are located at regions where the lightly stained euchromatin makes contact with the nuclear membrane. These pores presumably are the sites where messenger and transfer ribonucleic acids (RNAs) are transported out of the nucleus into the cytoplasm. Binucleate cells also are seen occasionally.
Mitochondria are elongate cylindrical structures that may appear oval in cross-section and may contain well-developed cristae and many matrix granules. They occur throughout the cytoplasm, among the granular endoplasmic reticulum or zymogen granules, and adjacent to the basolateral cell border. The cytoplasmic matrix occupies about 45% of the cell volume.34
Granular endoplasmic reticulum (see Fig. 55-7) occupies about 20% of the cell volume34,35 and fills most of the basal region of the acinar cells, although small amounts also occur in the apical region adjacent to and among the zymogen granules. This reticulum is composed of numerous parallel cisternal membranes covered with closely spaced attached ribosomes, giving the structures a granular appearance. On the basis of studies with laboratory animals, the ribosomes of the granular endoplasmic reticulum have been found to be the site of protein synthesis.36,37
The Golgi complex is located between the nucleus and the mass of zymogen granules present in the resting gland (see Fig. 55-7). It consists of flattened, membranous saccules as well as small vesicles or vacuoles that contain flocculent electron-dense material. The Golgi saccules have been distinguished from other intracellular vesicles by enzyme cytochemistry and by immunohistochemistry using antienzyme and antireceptor antibodies.38 The Golgi complex is believed to play an important role in the transport of secretory proteins and the formation of zymogen granules. The mechanisms by which these processes occur are still unresolved.
The secretory granules of the pancreas usually are divided into two types, electron-lucent condensing vacuoles and electron-dense zymogen granules. The condensing vacuoles are typically seen in the vicinity of the Golgi complex and, on the basis of autoradiographic data,36,37 are believed to be precursors of the zymogen granules. They are membrane-bound vesicles slightly larger than zymogen granules and much less numerous, occupying only about 2% of the cytoplasm.35 Zymogen granules also are spherical, membrane-bound vesicles, slightly less than 1 mm in diameter,28,39,40 filled with electron-dense material that apparently represents the digestive enzymes (see Fig. 55-7).
Studies of the chemical composition of the zymogen granules have shown that they contain about 12 to 15 different digestive enzymes, which make up about 90% of the granule protein.41–44 Each granule apparently contains the entire complement of secreted digestive enzymes, because labeled antibodies to several different enzymes have been located over single zymogen granules from different cells.45,46 Individual zymogen granules can differ markedly in the concentration of specific digestive enzymes contained within the granules.47 The digestive enzymes within the granules are not in solution or suspension but in a solid-state array, which exhibits specific binding between the enzymes themselves and between the enzymes and the granule membrane.48–51 Isolated zymogen granules are stable at slightly acid pH; at alkaline pH, they release their enzymes into solution.49,51,52
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree