Anal Canal Cancer
Cathy Eng
Jaffer Ajani
The treatment of squamous cell carcinoma (SCCA) of the anal canal is unique to gastrointestinal malignancies and depends solely on the efficacy of the combined modalities of chemotherapy and radiation therapy (XRT), reserving surgery for the management of residual or recurrent disease. This practice evolved from a systematic evaluation of a fortuitous observation in only three patients with SCCA of the anal canal, in which a treatment regimen intended as neoadjuvant chemoradiation prior to radical surgery proved sufficient of itself to completely eradicate their anal canal cancers (1). Additional studies have since confirmed the curability of SCCA of the anal canal with relatively low doses of radiation and chemotherapy. Although the current treatments have been largely successful, chemoradiation therapy may result in both acute and delayed treatment-related toxicities. However, the rarity of this cancer type has limited progress in the evaluation of its biological behavior and of proposals to further improve the treatment approach. The treatment concepts first developed in the 1970s have now been refined so that many patients are now not only cured but also retain anal sphincter function. Nevertheless, many questions are unanswered, and the optimum treatment schedules and chemotherapy agents, especially for larger or metastatic cancers, remain to be defined.
Anatomy
The anal canal consists of the terminal 3 to 4 cm of the intestine, extending from the rectum to the junction with the perianal skin (Fig. 47.1). The posterior wall of the canal is generally approximately 1 cm longer than the anterior wall. The superior limit of the anal canal is more readily appreciated by palpation than visually and is the upper border of the anorectal muscle ring (2). The anorectal ring corresponds to the junction of the puborectalis part of the levator ani muscle with the external anal sphincter. The distal limit, or anal verge, is the level at which the walls of the canal come into contact in their normal resting state. The perianal area is the skin within the 5-cm radius of the anal verge. The anal margin has been defined in a variety of ways, including as a synonym for the anal verge, or as the perianal skin immediately adjacent to the distal limit of the anal canal. Some physicians apply the term anal margin to the whole extent of the perianal skin.
The perianal skin is similar histologically to hair-bearing skin elsewhere. At the anal verge, the pigmented perianal skin blends with the paler, modified squamous epithelium of the distal canal, lacking hair or cutaneous glands, and is known as the pecten. The modified squamous epithelium of the pecten merges, usually just below the pectinate or dentate line, which marks the level of the anal valves, with a reddened membranous transitional zone that includes features of rectal, urothelial, and squamous epithelia. The transitional zone extends proximally for approximately 2 cm, where it blends with the pink columnar-glandular mucosa of the rectum. This last histologic change bears a variable relationship to the palpable level of the upper border of the anorectal muscle ring.
There are three major lymphatic pathways from the anal tissue, with numerous lymphatic connections between the various levels of the canal and anal verge. Lymphatics from the uppermost part of the canal drain to the perirectal and superior hemorrhoidal nodes of the inferior mesenteric system. Those from the area around and above the dentate line flow to the internal pudendal, hypogastric, and obturator nodes of the internal iliac system. Lymphatics from the distal canal, anal verge, and perianal skin drain to the superficial inguinal nodes, and occasionally to the femoral nodes, of the external iliac system.
The arterial supply to the distal rectum and anal canal is from the superior, middle, and inferior hemorrhoidal vessels, arising from the inferior mesenteric, internal iliac, and internal pudendal arteries, respectively. The venous drainage follows the arterial inflow, and thus accesses the hepatic portal and systemic venous systems.
The external anal sphincter is a voluntary muscle that is innervated by the internal rectal nerve, a branch of the pudendal nerve derived from the second, third, and fourth sacral nerves (S2, S3, and S4, respectively). In addition to its motor function, the internal rectal nerve also transmits pain, touch, and other sensations from the anal canal below the dentate line and from the perianal skin. The internal anal sphincter is an involuntary muscle, innervated by parasympathetic fibers from S2, S3, and S4, and by sympathetic fibers from the hypogastric plexus.
Incidence
Anal cancer remains one of the rarest malignancies of the gastrointestinal tract, affecting ≤1 per 100,000 persons (3), resulting in approximately 4,650 cases diagnosed and 690 deaths in 2007 (4). The majority of patients present with locally advanced disease and are treated with combined chemoradiation therapy with curative intent. The annual incidence is 1 per 100,000 in the heterosexual population but increases to 35 per 100,000 in men who have anal intercourse (5). Review of the Surveillance, Epidemiology, and End Results database between 1973 and 2000 indicates increasing incidence among both men and women, with black men having the highest incidence and worst prognosis relative to other races (6).
Risk Factors
Current literature suggests a strong link between cervical and anal cancer; it is believed that this is attributed to a history of
acquiring the human papillomavirus (HPV). It is believed that 20 million Americans ages 15 to 59 are currently infected with HPV or 1 in every 13 individuals, with 5.5 million new cases diagnosed per year. Approximately one-half of those individuals infected with HPV are adolescents, sexually active, and between the ages of 15 to 24 (7). As in cervical intraepithelial neoplasia, HPV infections may cause anal intraepithelial neoplasia (AIN). In addition to the relationship observed between HPV and intraepithelial neoplasia, HPV is also associated with the development of genital warts (condylomata acuminata), which can convert to SCCA after a prolonged latency period (5–40 years) (8,9). The prevalence of HPV appears to be bimodal with the greatest incidence occurring among individuals younger than 25 years of age, with a second peak after the age of 55 (10). In general, HPV infections are fairly common in young women, with the majority resolving spontaneously due to an effective immune response. However, persistent HPV infection may be the precursor for malignant transformation (9). More than 100 different subtypes of HPV exist; HPV subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, and 82 are considered to be the most carcinogenic, with subtypes 16 and 18 most frequently associated with anal cancer; and >70% of patients with invasive anal cancer are seropositive for subtype 16 (11). Controversy exists over whether having multiple subtypes of HPV in the same individual increases the persistence of HPV infection (12). At this time, the detection of HPV in patients with anal cancer does not appear to be a prognostic factor, but persistence of HPV infection may play a central role (13,14,15). The development of HPV is also linked to the less common anogenital cancers, including vulvar, vaginal, and penile cancers, as well as head and neck cancers of the oropharyngeal tract (16).
acquiring the human papillomavirus (HPV). It is believed that 20 million Americans ages 15 to 59 are currently infected with HPV or 1 in every 13 individuals, with 5.5 million new cases diagnosed per year. Approximately one-half of those individuals infected with HPV are adolescents, sexually active, and between the ages of 15 to 24 (7). As in cervical intraepithelial neoplasia, HPV infections may cause anal intraepithelial neoplasia (AIN). In addition to the relationship observed between HPV and intraepithelial neoplasia, HPV is also associated with the development of genital warts (condylomata acuminata), which can convert to SCCA after a prolonged latency period (5–40 years) (8,9). The prevalence of HPV appears to be bimodal with the greatest incidence occurring among individuals younger than 25 years of age, with a second peak after the age of 55 (10). In general, HPV infections are fairly common in young women, with the majority resolving spontaneously due to an effective immune response. However, persistent HPV infection may be the precursor for malignant transformation (9). More than 100 different subtypes of HPV exist; HPV subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, and 82 are considered to be the most carcinogenic, with subtypes 16 and 18 most frequently associated with anal cancer; and >70% of patients with invasive anal cancer are seropositive for subtype 16 (11). Controversy exists over whether having multiple subtypes of HPV in the same individual increases the persistence of HPV infection (12). At this time, the detection of HPV in patients with anal cancer does not appear to be a prognostic factor, but persistence of HPV infection may play a central role (13,14,15). The development of HPV is also linked to the less common anogenital cancers, including vulvar, vaginal, and penile cancers, as well as head and neck cancers of the oropharyngeal tract (16).
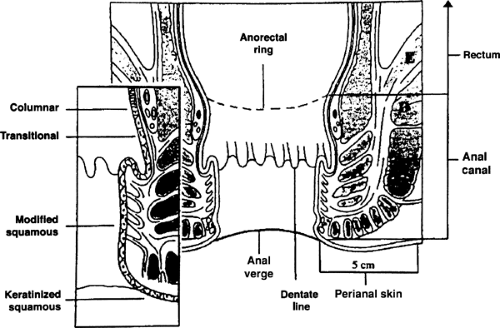 FIGURE 47.1. Anatomy of the anal canal. Source: Adapted from Skarin AT, ed. Atlas of Diagnostic Oncology. New York, NY: Gower Medical Publishing; 1991;3:43 . |
Other infectious diseases linked to the pathogenesis of anal cancer include herpes simplex virus (HSV), chlamydia trachomatis, gonorrhea, and other sexually transmitted diseases (17). In addition, an increased risk of developing anal cancer has been shown in men and women with a history of receptive anal intercourse prior to age 30 years; having had sexual intercourse with more than 10 partners; chronic immunosuppression; and/or having had a history of cervical, vulvar, or vaginal cancers (18).
The precise role of HIV infection as a causative agent in the development of anal carcinoma is still undefined. It has been observed that patients who are HIV positive are two to six times more likely than HIV-negative individuals to have anal HPV, regardless of sexual practices (19). In addition, HIV-positive patients are more likely to have persistent HPV infection and are two times more likely to progress from low-grade AIN to high-grade lesions, as compared with HIV-negative individuals. In fact, an inverse correlation with persistence of HPV infection and progression from low-grade to high-grade AIN has been shown to be related to the CD4 lymphocyte count (19).
Several reports suggest a direct correlation between HIV infection and anal cancer. In New York City between 1979 and 1985, the incidence of anal cancers increased 10-fold among men who were 20 to 49 years of age, coinciding with the appearance of AIDS (20). It has been estimated that men who are 25 to 44 years old are 60-fold more likely to die from anal cancer if they are HIV positive as compared to HIV-negative individuals (21). Melbye et al. showed that AIDS patients in the United States had a 64-fold higher relative risk of developing anal cancer as compared to the general population (22,23). In contrast to these data, prior studies have failed to demonstrate any direct association between AIDS and anal cancer. Data from analysis of single men in San Francisco during the 1980s demonstrated that the incidence of anal cancer increased during this time period but did not necessarily correlate with HIV status (24). It is clear, however, that HIV-positive patients are immunosuppressed and are predisposed to HPV infection and AIN, which have been shown to be involved in the development of anal cancer. Hence, anal carcinoma is considered an AIDS-associated malignancy and is not considered an AIDS-defining illness. Unexpectedly, the advances in the treatment of HIV patients with highly active antiretroviral therapy (HAART) has prolonged the survival of this patient population; yet, after the introduction of HAART, the prevalence of AIN has remained unchanged among HIV-positive men having sex with men (MSM) (25).
As in HIV-positive patients, a state of chronic immunosuppression (e.g., organ transplant patient) places the patients at high risk for developing HPV infection and subsequent development of AIN (26). It is reported that organ transplant patients have a 10-fold increase of developing carcinoma of the anal canal (27). Last, a history of tobacco use has been shown to increase the risk of anal cancer by a factor of two to five times, independent of sexual practice, and this risk appears to
be greater in premenopausal than in postmenopausal women (28).
be greater in premenopausal than in postmenopausal women (28).
Histopathology
Approximately 90% of primary cancers of the canal are of squamous cell histology (SCCA). The major subtypes are large cell keratinizing, large cell nonkeratinizing (transitional), and basaloid (29). The lack of prognostic differentiation for these various subtypes has largely led to a general category of SCCA of the anal canal. The remaining 10% of malignant anal canal cancers include adenocarcinoma of the anal glands, small and large cell cancers, and melanoma. Primary cancers of the perianal skin or anal margin are similar to cancers of the skin in other sites. The majority are SCCAs, with occasional basal cell cancers and melanomas.
Presentation and Diagnosis
Most patients present with nonspecific symptoms. Bleeding, anal discharge, discomfort, itching, or pain is reported by approximately half of all patients with cancers of the canal; approximately 25% of patients are aware of a mass. Other patients may present with enlargement of their inguinal lymph nodes (30). Anal tumors are typically plaquelike or ulcerated and rarely pedunculated. Benign conditions, such as hemorrhoids, fibrous skin tags, redundant mucosa, and anal fissures, may make the diagnosis difficult. An incidental discovery of superficial cancer or high-grade dysplasia is sometimes identified after histologic examination of tissue removed during treatment for benign conditions such as hemorrhoids or chronic fissures. Gross fecal incontinence due to sphincter incompetence or fistula formation is uncommon and is seen in <5%. Extrapelvic metastases are a rare presenting indication of anal cancer and are diagnosed in <5% of patients at presentation (3); common sites include the liver, lungs, and bones. More than two-thirds of patients are diagnosed at a relatively early stage, before lymph node metastases or invasion of adjacent organs become clinically detectable (31).
Diagnostic Workup and Staging
Unless stated otherwise, the remainder of the chapter focuses primarily on SCCA. The features of greatest prognostic significance for diseasefree survival (DFS) are the size of the primary cancer, spread to regional lymph nodes, and extrapelvic sites. The probability of retaining anal function is determined principally by sphincter competence at presentation and the size of the primary cancer. Inguinal lymph node metastases are clinically detectable in approximately 15% of patients when the primary cancer is first diagnosed. In series managed by radical surgery, pelvic node metastases were found in approximately 30%, with approximately equal risk of involvement in the internal iliac and perirectal-superior hemorrhoidal node pathways (32,33).
The primary tumor and any clinically palpable inguinal nodes should be biopsied to establish the diagnosis. Abdominal and pelvic computed tomography (CT) scans or magnetic resonance imaging may disclose liver and nodal metastases, but small nodal metastases are not identified reliably by any currently available imaging technique, including transrectal ultrasound. A digital rectal examination, as well as anoscopy, proctosigmoidoscopy, or flexible sigmoidoscopy, should be done for a complete evaluation. A standard chest x-ray or CT scan of the chest is sufficient as a screen for pulmonary metastases. Localized skeletal symptoms should also be evaluated radiologically, but screening bone scans are not necessary in asymptomatic patients. Fluorine-18 2-fluoro-2-deoxy-D-glucose-positron emission tomography may also have a role in staging but is not advocated by all clinical investigators (34,35,36). Full blood count, kidney and liver function tests, and, if risk factors are present, HIV antibody tests, including CD4 count and viral load, should be performed. Unlike other gastrointestinal malignancies such as colorectal and pancreatic cancer, a tumor marker for SCCA of the anal canal has not been identified.
The current American Joint Committee on Cancer (AJCC) (37) and International Union Against Cancer (UICC) staging systems (38) are based on the size of the primary cancer and the presence or absence of regional lymph node or distant metastases (Table 47.1). Unlike other malignancies where the degree of tumor penetration is indicative of the T stage, the T stage of anal cancer is primarily based on the size of the tumor.
Surgery as the Primary Modality of Therapy
Prior to the discovery of combined chemoradiation therapy as a treatment modality with curative intent, the best treatment modality appeared to be an abdominoperineal resection (APR). As a single treatment modality, the estimated 5-year probability of survival is 40% to 70% (32). APR is a major surgical procedure involving en bloc resection of the rectum and the anal canal. The mesorectum is dissected from the posterior and lateral pelvic sidewalls distal to the aortic bifurcation, and the dissection includes the levator muscles and muscles of the pelvic floor. The final specimen includes the anorectum and mesorectum, together with the pelvic floor muscles, ischiorectal fat, and perianal skin. An end sigmoid colostomy is fashioned. Where necessary, the dissection is extended to include the posterior vaginal wall and, occasionally, other pelvic organs, such as the uterus, ovaries, prostate, or bladder, resulting in a pelvic exenteration. Despite the curative intent of surgery, the possibility of a permanent colostomy can be socially and emotionally distressing for patients. Some patients may be so overwhelmed with the possibility of an APR that they delay treatment, placing them at risk for further disease advancement.
With the widespread adoption of combined radiation and chemotherapy as the initial treatment for anal cancer, the role of surgery has changed. Local excision is now generally reserved for patients who have SCCA tumors up to 2 cm in diameter (T1) and are superficial and well differentiated. The risk of nodal metastases is <10% for small, well-differentiated cancers that have not penetrated into the sphincter muscles. Local resection can also be considered for small cancers discovered at the time of surgery for benign anal conditions. Patients who fail local resection can be assessed for combined chemoradiation treatment if further local excision is not indicated.
The main role for APR is now reserved for surgical salvage of patients who have residual or recurrent cancer. An APR may also be indicated for patients who have contraindications to radiation or chemoradiation therapy. If residual cancer is suspected after completing chemoradiation and an adequate period has elapsed for the full treatment effects of XRT, it is advisable to confirm the presence of active cancer histologically with a tissue biopsy before proceeding with salvage surgery. The effectiveness of surgical salvage varies considerably, although several series have reported control rates of approximately 50% to 60% (39,40).
Table 47.1 Tumor, Node, Metastasis (TNM) Classification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Single-Agent Radiation Therapy
XRT is another option of preservation of the anal sphincter in lieu of surgery with evidence for long-term survival. When compared to APR, it has been reported that the overall survival (OS) is equivalent, with a complete response (CR) achieved in approximately 75% of patients (41,42). A large retrospective study completed by Deniaud-Alexandre et al. evaluated 305 patients treated with curative intent radiotherapy (RT) (43). The median dose of external beam radiation was 45 Gy. A radiation boost of 20 Gy was provided after a median delay of 37 days (median cumulative dose of 63 Gy) in 279 patients (92%). All palpable lymph nodes received a booster dose of 10 to 15 Gy. After a median follow-up of 103 months, a complete response was achieved in 79% to 96% of T1–3 tumors but in only 44% for T4 tumors. Overall, XRT resulted in local regional control in only 68% of patients: T1–2 (78%–81%), T3 (63%), and T4 (33%). Tumor size ≥4 cm and a treatment delay of more than 38 days were both negative prognostic factors in patient outcome.
Uninterrupted courses of high-dose radiation, delivering 50 Gy in 4 weeks or 60 to 65 Gy in 6 weeks may control 80% or more of cancers up to approximately 4 cm in size and approximately 50% of larger tumors. Papillon et al. reported the success of the split-course regimen, in which an intensive course of small field external beam radiation is directed to the perineum (42 Gy in 10 fractions in 16 days), and followed 8 weeks later by 20 Gy interstitial radiation (44).
Radiation alone may be favored by some for the treatment of T1 category cancers (≤2 cm) and may be curative (45). Although the use of radiation alone in these situations spares the patient side effects of chemotherapy, the higher doses required when radiation is used alone may increase the risk of normal tissue damage (46). Some surgeons may consider local excision of small cancers followed by adjuvant XRT (47).
Treatment of Anal Canal Cancer with Combined Radiation and Chemotherapy: The Classic Approach
Clinical investigators proceeded to seek other options to enhance the efficacy of XRT. In 1974, Nigro et al. reported that treatment with concurrent radiation, 5-fluorouracil (5-FU), and mitomycin C (MMC) produced durable, complete regression of squamous cell cancers of the anal canal (1). After confirmation by numerous other clinicians, concurrent chemo radiation has replaced radical RT alone or surgery as the preferred initial treatment for most patients with anal canal cancer. Three major randomized trials have demonstrated that (a) delivering 5-FU and MMC concurrently with radiation gives outcomes superior to those of the same schedule of radiation alone (48,49) and (b) the combination of 5-FU and MMC with radiation is more effective than 5-FU with radiation (50).
In a trial by the United Kingdom Coordinating Committee for Cancer Research (UKCCCR), 577 patients with all stages of epidermoid cancer of the anal canal (75%) or anal margin (23%) were enrolled (48). Twenty percent were lymph node positive, and 2.5% had extrapelvic metastases. They were randomized to treatment with either radiation alone (45 Gy in 20–25 fractions in 4–5 weeks) or radiation with 5-FU (1,000 mg/m2 per 24 hours for 96 hours or 750 mg/m2 per 24 hours for 120 hours) by continuous intravenous (IV) infusion during the first and final weeks of radiation treatment, and MMC (12 mg/m2) by bolus injection on day 1 of the first course of 5-FU only. Patients with other comorbidities or those older than 80 were provided a reduced dose of 5-FU (750 mg/m2, D1–D4) and MMC (10 mg/m2, D1). Six weeks after the initial phase of treatment, patients received additional radiation without chemotherapy (15 Gy in six fractions by external beam
therapy or 25 Gy over 2 to 3 days by iridium-192 implant). For those patients whose tumor showed less than a clinical partial response (defined as shrinkage of <50%) at 6 weeks post treatment, radical surgery was performed rather than additional radiation. The definition of local failure in this trial included the presence of residual or recurrent cancer in the primary site or regional nodes, treatment-related morbidity requiring surgery, or inability to close a colostomy that was created prior to treatment. Surgery with colostomy was required for late treatment-induced toxicity in 10 patients (3.5%) in each study arm. Six patients (2%) in the combined modality group and two patients (0.7%) in the radiation alone group died of treatment-related morbidity. Local control and cause-specific survival rates were significantly improved and the need for colostomy decreased by combined modality treatment (Table 47.2). The OS rate was improved but was not statistically significant.
therapy or 25 Gy over 2 to 3 days by iridium-192 implant). For those patients whose tumor showed less than a clinical partial response (defined as shrinkage of <50%) at 6 weeks post treatment, radical surgery was performed rather than additional radiation. The definition of local failure in this trial included the presence of residual or recurrent cancer in the primary site or regional nodes, treatment-related morbidity requiring surgery, or inability to close a colostomy that was created prior to treatment. Surgery with colostomy was required for late treatment-induced toxicity in 10 patients (3.5%) in each study arm. Six patients (2%) in the combined modality group and two patients (0.7%) in the radiation alone group died of treatment-related morbidity. Local control and cause-specific survival rates were significantly improved and the need for colostomy decreased by combined modality treatment (Table 47.2). The OS rate was improved but was not statistically significant.
Table 47.2 Three-Year Results of Randomized Trials of Radiation Alone versus Radiation, 5-Fluorouracil, and Mitomycin C | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||
The European Organization for Research and Treatment of Cancer (EORTC) performed a similar study of only 103 patients with advanced cancers of the anal canal (49). Patients with extrapelvic metastases and those older than 76 years of age were ineligible. Eighty-five percent of those entered had stage T3–4 cancers, and 51% had abnormal nodes. Patients were randomized to pelvic radiation treatment (consisting of 45 Gy in 25 fractions over 5 weeks) alone or with 5-FU (750 mg/m2 per 24 hours for 120 hours by continuous IV infusion) during weeks 1 and 5 of radiation. In the first week only, a bolus injection of MMC (15 mg/m2) was given on the first day of the 5-FU infusion. Six weeks later, additional radiation was delivered by external beam or interstitial techniques (15 Gy if there had been complete clinical response to the initial course of treatment, 20 Gy if response had been partial). Inadequate response resulted in surgery in 5 patients on the radiation only arm. Acute and late toxicity rates were similar in each treatment group. One of 51 (2%) patients who received radiation and chemotherapy died of treatment-related toxicity. The local control and colostomyfree survival (CFS) rates were significantly better after combined modality but, as in the UK trial, the improvement in OS rates did not reach statistical significance (Table 47.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







