Chapter 67 Amebiasis and other parasitic infections
Amebic Liver Abscess
History
The earliest report of amebiasis is probably the Sanskrit document Brigu-samhita, written about 3000 bce, referring to bloody mucoid diarrhea (Vaidya & Ray, 1982). Assyrian and Babylonian texts refer to blood in the feces, suggesting the presence of amebiasis in the Tigris-Euphrates basin before the sixth century bce (Bray, 1996), and it is possible that hepatic and perianal abscesses described in both Epidemics and Aphorisms in the Corpus Hippocratorum refer to amebiasis (Jones et al, 1948; Jones & Whithington, 1953). After the death of Hippocrates in 356 bce, Alexander the Great became king of Macedonia. In Alexander’s eastern campaign, he reached an area where amebiasis was endemic, and he died on the return trip at the age of 33 years, probably of an amebic liver abscess (Saville, 1964).
In 1818, Ballingall described a surgical technique to drain liver abscesses. When Ballingall practiced in Madras, India, Napoleon Bonaparte was exiled on the tropical island of St. Helena by the British after his defeat at Waterloo, where bloody diarrhea—possibly amebiasis—was common. Napoleon died in 1821 after an attack suggestive of amebic liver abscess, with symptoms of fever, diarrhea, and painful, tender swelling in the epigastrium. At the postmortem, an ulcer was found at the small curve of the stomach, communicating with the liver, suspected to be due to rupture of an amebic liver abscess of the left lobe into the stomach (Chaplin, 1913). In 1828, James Annesley gave detailed descriptions of “hepatic dysentery” (Kapoor, 1979). The connection between amebic dysentery and liver abscesses was described by the English physician William Budd (1857), but Charles Morehead, professor of Medicine and first principal of Grant Medical College, Bombay, India, was the first to report a case of hepatic abscess in 1848 (Martinez Baez, 1986).
Entamoeba histolytica was discovered by Friedrich Lösch in 1873 in Russia (Lösch, 1975). Lösch recognized amebae in the colon and terminal ileum accompanying acute dysentery (Martinez Baez, 1986). He gave descriptions of amebae, including structure, size, motility, intracytoplasmic elements, and drawings. Lösch named the amebae after his patient; Amoeba coli was proved later on sequencing of the genome (Tovar et al, 1999), and a calreticulin-like protein and Golgi apparatus were detected in the amebae (Gonzalez et al, 2002).
Stephanos Kartulis, a Greek physician, found amebae in intestinal ulcers in patients from Egypt in 1885 and noted that he never found amebae from nondysenteric cases (Kartulis, 1886). In 1890, Osler reported a young physician who died of amebic liver abscess (ALA) after an attack of dysentery. The report by William Thomas Councilman and Henri Lafleur, working at Johns Hopkins in 1891, represents a definitive statement about the pathology of amebiasis, amebic dysentery, and ALA at the end of the nineteenth century (Faust, 1931). Schaudinn did differentiate between harmless Entamoeba coli and pathogenic E. histolytica (Martinez Baez, 1986). In 1901, Harris produced ALAs by intrarectal infection of puppies with E. histolytica. Musgrave and Clegg (1904) cultivated E. histolytica in vitro, introducing the term amebiasis. In 1952, Hoare reported that E. histolytica had three phases. Species of amebae found in human host; E. gingivalis, Entamoeba coli, Iodamoeba bütschlii, and E. hartmanni were discovered between 1849 and 1919 (Martinez Baez, 1986).
A plant alkaloid, concessine, was found to kill E. histolytica (Vaidya & Ray, 1982). The first effective treatment came from Brazil in the form of ipecac; emetine was isolated from ipecac in the nineteenth century. Leonard Rogers (1912), professor of pathology at Medical College Hospital in Calcutta, India, reported successful treatment of both intestinal and hepatic amebiasis by injectable salts of emetine. The 1930s witnessed the introduction of two important hydroxyquinolines introduced by Anderson and Koch in 1931 and by a number of others. Although largely replaced by imidazoles in the 1980s, hydroxyquinolines remain useful today. In 1966, Powell and his colleagues demonstrated the effectiveness of metronidazole as an amebicidal agent in both intestinal and extraintestinal amebiasis.
Epidemiology
Approximately one tenth of the world population is believed to be infected with E. histolytica, with 100,000 deaths worldwide each year due to invasive amebiasis (Haque et al, 2003; Petri et al, 2000; Walsh, 1986; World Health Organization [WHO] et al, 1997b). Amebiasis is the third most common parasitic cause of death worldwide (Li & Stanley, 1996). The prevalence of E. histolytica could be an overestimate because it dates before the separation of E. histolytica from the morphologically identical, nonpathogenic E. dispar (Diamond & Clark, 1993) and E. moshkovskii (Beck et al, 2008; Fotedar et al, 2008). However, recent prevalence and morbidity data obtained through molecular techniques allow construction of more reliable map of endemic regions of amebiasis around the world, such as on the Asian subcontinent (India, Bangladesh), Africa, Asian Pacific (Thailand, Japan), and South and Central America (Mexico, Colombia) (Ximenez et al, 2009).
Disease expression varies geographically; invasive disease seen in Egypt, predominantly amebic colitis, may vary in schistosomiasis-endemic versus nonendemic areas (Mansour et al, 1997), whereas in South Africa an excessive rate of amebic liver abscess is noted. Although amebic liver abscess has been encountered in epidemic form in temperate climates, as it was in the water-borne epidemic in Chicago in 1933 (Munoz, 1986), it is a problem chiefly of the tropics and developing countries (Sepulveda, 1982). In developed countries, it continues to be encountered sporadically in immigrants or travelers from endemic zones, low socioeconomic groups, residents of institutions, and male homosexuals (Ravdin & Stauffer, 2005).
In the United States and Europe, homosexual males are principally colonized with E. dispar, and patients infected with the human immunodeficiency virus (HIV) had no increased risk of either intestinal or extraintestinal amebiasis (Petri & Singh, 2006). Invasive extraintestinal amebiasis (hepatic abscesses) are more frequent in HIV-infected patients in these countries (Hung et al, 2005), whereas the same is not true elsewhere (Moran et al, 2005). E. histolytica remains a significant cause of morbidity and mortality in developing countries, where infection is common through flies and food handlers. The annual incidence of amebic dysentery in children is 2.2% in Bangladesh (Haque et al, 2003), and annual incidence of amebic liver abscess averaged 21 cases per 100,000 inhabitants in Hue City, Vietnam (Blessmann et al, 2002). Ingestion of E. histolytica occurs where poverty facilitates deficiencies in sanitation and sewage treatment. In addition, a recent report suggests that parasite genotype plays a role in determining outcome of infection by E. histolytica (Ali et al, 2007).
Organism
E. histolytica is a protozoan with two forms: trophozoite and cyst. Cysts constitute the infective form through fecal-oral transmission via food, water, or direct person-to-person contact. Cysts survive the acid of the stomach and travel through the small intestine, and within the terminal ileum or colon, trophozoites emerge to complete the life cycle (Guerrant, 1986). Cysts can survive for 45 minutes in feces lodged under fingernails and for 1 month in soil at 10° C. They remain infective in fresh water, seawater, and sewage but are destroyed by drying, iodine, and heat. They are not killed by chlorination used to purify drinking water (Munoz, 1986). The genus Entamoeba includes the pathogenic species E. histolytica and nonpathogenic E. hartmanni, E. coli, E. polecki (in swine), and E. moshkovskii (from sewage) (Guerrant, 1986). E. histolytica is morphologically similar to E. dispar; therefore the study of zymodemes, patterns of the electrophoretic mobility of isoenzymes; genetic differences using RNA and DNA probes; and the use of polymerase chain reaction (PCR) amplification became more reliable in their identification (Tannich et al, 1989). Encoding genes for transcription factors have been cloned for E. histolytica (Castanon-Sanchez et al, 2009).
Community Screening Procedures
An effective community screening protocol includes 1) microscopic examination of unfixed fecal samples, 2) Ritchie’s fecal concentration, 3) staining of alcohol-fixed stools, 4) Robinson’s in vitro culture, 5) screening stool antigens, 6) serology (indirect hemoagglutination test [IHAT], enzyme-linked immunosorbent assay [EIA], and immunofluorescence test [IFT]), and 7) isoenzyme electrophoresis of stool for zymodeme identification (Thomas & Garg, 2007).
Direct microscopic examination is found to be more sensitive than serology; however, the sensitivity of both is lower than Robinson’s culture and zymodeme identification, the gold standards (Gatti et al, 2002). DNA-based PCR is rapid and sensitive to detect cysts in the stool (<5) and in fluids aspirated from amebic liver abscess (Rivera et al, 1998). A PCR test for E. moshkovskii was developed with a high sensitivity and specificity using DNA from stool samples (Fotedar et al, 2007). In 2007, Helmy used nested PCR and restriction enzyme digestion (RED) to distinguish E. histolytica from E. dispar.
Host Factors
The major reservoir for Entamoeba spp. is the human being. Male homosexuals transmit the infection but usually harbor nonpathogenic E. dispar, which is more common in females (Gatharim & Jackson, 1987). E. histolytica can be transmitted by heterosexual activity as well as male and female homosexual activity (Salit et al, 2009), although menstruating women are protected against invasive infection. Breastfed neonates have a low incidence as a result of the presence of immunoglobin A (IgA) and low iron content in breast milk (Thomas & Ravindra, 2000). A diet rich in iron and carbohydrates predisposes to invasive amebiasis (Gatharim & Jackson, 1987), and HLA-DR3 gene expression is an independent risk factor for amebic liver abscess (Arellano et al, 1996).
Although immunosuppression is considered an important risk factor for invasive amebiasis in Asian Pacific countries (Hsu et al, 2008; Hung et al, 2008), others maintain there is no particular susceptibility for developing invasive forms of amebiasis in immunosuppressed individuals. Nevertheless, some HIV infections have been detected during admission of amebic liver abscess patients. On the other hand, the natural history of the disease seems to be the same as in nonimmunosuppressed patients (Kershenobish & Corona, 2008).
Pathogenesis
The disease course is determined by three virulence factors: lectin (a surface protein), amebapores (small peptides), and cysteine proteases. Trophozoite adhesion to the colonic wall is mediated by lectin, which results in persistent infection, and caspase 3 activation, which is a crucial step in cell necrosis and abscess formation (Huston et al, 2003). Amebapores are inserted by the trophozoite into the host cell, where they puncture the lipid bilayer and form a portal of entry into the host. Amebapores result in colloid osmotic lysis of the cell (Leippe et al, 1991). Cysteine proteases contribute to degradation of the extracellular matrix proteins and disruption of cell monolayers (Que & Reed, 1997).
It is anticipated that antiamebic antibodies protective against invasive infection would block lectin binding and neutralize amebapore and cysteine proteases. It has been suggested that the proteophosphoglycans (PPGs) in the amebic glycocalyx may participate in E. histolytica pathogenicity because the closely related, nonpathogenic E. dispar lacks a significant glycocalyx surface layer (Bhattacharya et al, 2000). Furthermore, antibodies that bind to PPGs neutralize liver abscess formation (Marinets, 1997). PPGs are anchored into the parasite cell membrane by a glycosylphosphatidylinositol (GPI) moiety. Synthesis of the GPI anchor requires a cascade of enzymes, including mannosyl transferase 1 (PIG-M1), whose blockage reduces GPI synthesis and PPGs in trophozoites (Weber, 2008). In addition, experimental evidence suggests that liver cell necrosis is increased when neutrophils are present along with E. histolytica (Aikat et al, 1979).
Normal blood flow in the portal vein is about 1.4 L/min, blood pressure is 12 to 15 mm Hg, and erythrocyte velocity is 8 to 18 cm/s. In comparison, sinusoidal blood flow is extremely low (3.4 to 0.16 mL/min), as is red blood cell velocity (0.1 mm/s; Puhl et al, 2003). Forces exerted on a parasite that adheres to the endothelium are thus much lower in the sinusoids and may partly explain why the parasite crosses the endothelium within these structures. Lack of tight junctions in liver sinusoidal endothelial cells (LSECs) can facilitate crossing by the parasite, creating a larger breach when reaching the hepatic parenchyma (Blazquez et al, 2007).
Publication of the E. histolytica genome (Loftus et al, 2005) facilitated transcriptome studies of the parasite. Microarrays have been used to compare virulent and avirulent trophozoites (those unable to form liver abscesses) from the same strain (Santi-Rocca et al, 2008). Overexpression of peroxiredoxin and rubrerythrin genes in virulent parasites is of interest for ALA development because inflammatory cell recruitment and subsequent inflammation are features of liver infection by E. histolytica (Choi et al, 2005a).
Molecular Genetics
The Institutes for Genomic Research have recognized that E. histolytica has a small, highly repetitive genome rich in adenosine-thymidine and densely packed sequences, but it lacks introns (Mann, 2002). Although some regions of the genome encode highly conserved proteins, other areas exhibit high degrees of polymorphism (Haghighi et al, 2002). Sequencing of the E. histolytica genome has revealed at least 44 genes (Clark et al, 2007). The purification of trophozoites from different organs of the same patients revealed that their tropism was linked to different genotypes (Ali et al, 2007).
Host Defense and Potential for Vaccine Development
It has not been definitively established which mechanism is responsible for invasion or recurrence (Ravdin & Guerrant, 1982). The first line of defense is the innate immunity that recognizes pathogen-associated molecular patterns (PAMPs) that trigger an inflammatory response. Natural killer T cells activated by E. histolytica are important in the control of amebic liver abscess (Lotter et al, 2009). Interferon (IFN)-γ initiates inflammation through macrophage production of tumor necrosis factor (TNF) and nitrous oxide (NO) synthesis by polymorphonuclear cells and macrophages. In vitro, the effects of IFN-γ can be bypassed by the recognition of PPGs of E. histolytica by Toll-like receptors (TLR) 2 and 4, which results in direct production of TNF and interleukins (ILs)-12 and -8 (Maldonado-Bernal, 2005). This shows the importance of early recognition of PPGs and inflammatory cell recruitment during ALA onset (Ivory & Chadee, 2007).
Complement cannot prevent invasion because it is absent from gut mucosal secretions (Braga et al, 1992), and amebic cysteine proteases degrade C3a and C5a (Reed et al, 1995). Neutrophils fail in initial host defense (Salata et al, 1989), and trophozoites can infect the human liver for months before abscesses are diagnosed. The second line of adaptive immune response constituted by activated lymphocytes and macrophages is the important effector mechanism against E. histolytica (Vohra et al, 2003). E. histolytica infection elicits mucosal IgA and serum IgG response to lectin protein shown to be protective against infection (Abd-Alla et al, 2004; Haque et al, 2002) in addition to epitope-specific antibodies that inhibit adherence to target cells (Pillai et al, 1999). The galactose/GalNAc lectin isolates from three distinct areas of the world—Bangladesh, Republic of Georgia, and Mexico—retain remarkable sequence conservation (Beck et al, 2002), and is recognized as a potential vaccine target (Thomas & Garg, 2007). Serum immunoglobulin (Ig) G response to the lectin protein does not provide protection against infection (Haque et al, 2002). Oral vaccines that use amebic antigens have been developed and tested in animals (Lotter et al, 2003; Mann et al, 1997; Snow & Stanley, 2006). A codon-optimized DNA vaccine has been tested in a murine model and was found to be useful in stimulating type 1 cellular immune response and serum antibodies (Gaucher & Chadee, 2002). Cell-mediated immunity (CMI) may be sufficient for vaccine protection from intestinal amebiasis with significant IFN-γ, interleukin (IL)-2, IL-12, IL-10, and IL-17 production with recombinant vaccines (Guo et al, 2009). Gram-negative bacteria expressing E. histolytica antigens may constitute a suitable oral vaccine carrier against invasive ameobiasis (Lotter et al, 2008).
Pathology
The development of ALAs is a fatal feature of infection by E. histolytica and is the most common extraintestinal form of invasive amoebiasis. Indeed, an estimated 100,000 people succumb to ALA each year (WHO, 1997a). Trophozoites that successfully penetrate the colonic mucosal barrier cause invasive disease, enter the portal system, and travel to the liver. Amebic colitis and amebic liver abscess rarely occur simultaneously, and the colonic lesions are usually silent; direct extension to the liver and lymphatic spread do not occur. The cecum is the most common site of amebic colitis, and the right lobe of the liver is more commonly affected because of drainage of the right portal branch from the right side of the colon. The condition usually starts as diffuse amebic hepatitis; liver cells undergo liquefactive necrosis, starting in the center and spreading peripherally to produce a cavity full of blood (Fig. 67.1) and liquefied liver tissue resembling anchovy sauce; it has no odor and is sterile. The fluid itself is free from any amebae, which may be found at the expanding edge of the abscess cavity with little inflammation. Amebae are known to lyse neutrophils, and the release of neutrophilic mediators may promote hepatocyte death and extension of the abscess. Secondary bacterial infection may occur spontaneously, altering the color, odor, and consistency of the pus. Lack of fibrotic response by the surrounding tissue with centrifugal extension results in extention of the abscess to the Glisson capsule, which is resistant to the amebae. Typically, amebic liver abscesses are solitary, large, and located in the right liver. Left lobe abscesses are less common, but because of the smaller volume of the left liver, abscesses in this location are more prone to rupture the capsule (Thomas & Ravindra, 2000). Vascular and biliary structures may traverse the abscess cavity; because of the intrahepatic covering of the Glisson capsule, such structures are resistant to the process of liquefactive necrosis. However, these structures can be mistaken for septa within the abscess cavity, and fracturing of these strands can lead to hemorrhage or biliary leak, or it can create a communication between the vascular and biliary channels and result in hemobilia and jaundice (Singh et al, 2008). The abscess wall is typically ill defined with a minimal host response of fibrous tissue, but longstanding abscesses may develop a fibrous wall and may even calcify (Rogers et al, 1980). In treated cases, complete resolution is the rule, but it may take 6 months to 2 years or longer (Thomas & Ravindra, 2000) than the usual time for pyogenic abscesses to resolve (Sudhamshu & Sharma, 2009).
Clinical Presentation
The peak incidence of amebic liver abscess is between 20 and 60 years of age; thus it is predominantly a disease of young men. Amebic liver abscess is 10 times more common in adults than in children and is three to 10 times more common in males (Sepulveda & Manzo, 1986; Wells & Arguedas, 2004). Population groups that are not commonly affected—such as children (especially neonates), pregnant women, and women in the postpartum period—have an increased risk of severe disease and death. Treatment with steroids, malignancy, men having sex with men, advanced age, and malnutrition could be considered risk factors for severe disease (Guerrant, 1986; Li & Stanley, 1996).
Most E. histolytica infections are asymptomatic or present as mild, “noninvasive” disease. Asymptomatic carriers, or cyst passers, may excrete cysts for a short period, but the majority of these patients clear the infection within 12 months. Patients with confirmed E. histolytica infection, even if they are asymptomatic, should be treated to eliminate the organism and prevent further transmission (Stanley, 2003). The time between penetration of colonic mucosa and damage to hepatic parenchyma is unknown. Active diarrhea usually occurs in less than 30% of patients at any time before presentation despite intestinal infection by E. histolytica. In most cases, standard stool microscopy results are negative, but in research studies, cultures of stool were positive for E. histolytica in more than 75% of patients with amebic liver abscess (Irusen et al, 1992).
Concomitant hepatic abscess is found in only one third of patients with amebic colitis. The duration of symptoms is usually 10 days. In nonendemic regions, such as Western Europe and the United States, patients usually report travel to an endemic area in the previous 2 to 5 months (median, 3 months), although a prolonged latency may occur (Johnston et al, 2009; Wells & Arguedas, 2004). Patients with amebic liver abscess start reporting nonspecific symptoms such as anorexia, nausea, vomiting, and acute colitic illness. Abdominal pain and fever are the cardinal symptoms of the disease, seen in 90% of patients or more. Fever typically is between 38° C and 40° C and is seen in 87% to 100% of patients; rigors occur in 36% to 69%. Other signs and symptoms vary according to the site and the size of the abscess. The chief sympton is typically the abrupt onset of right hypochondrial pain radiating to the right shoulder and scapular area. The pain increases with coughing, deep breathing, and walking. If the abscess is in the left hepatic lobe, the pain may be epigastric, precordial, or retrosternal and may radiate to the left shoulder. Abscesses located on the inferior aspect of the liver may present in a manner similar to peritonitis resulting from any upper abdominal cause. On occasion, the presentation is insidious, lasting 2 or more weeks; in such patients, significant weight loss may occur (Thomas & Ravindra, 2000). One report has suggested that amebic liver abscesses can be silent and asymptomatic (Blessmann et al, 2003).
Abdominal examination usually reveals a tender, soft hepatomegaly accompanied by overlying muscle guarding and intercostal tenderness. On occasion, increased warmth and cutaneous edema may occur. Jaundice is often seen, although it was previously reported to be a prominent feature in only 5% to 8% of patients (Pitt, 1990); biliary communication of amebic liver abscess has been reported in up to 27% (Agarwal et al, 1995; Bayraktar et al, 1996). Because the right posterior-superior surface is the most common site of amebic liver abscess, it is always accompanied by right basal lung signs as a result of either pleural effusion, empyema, or lung abscess (Loulergue & Mir, 2009). On the other hand, left lobe abscess may be complicated by pericardial friction, and abscesses in this location can extend into the pericardium, a sign associated with a very high mortality rate (Wiwanitkit, 2008). Hepatic failure, ascites, and splenomegaly may occur in 15% of patients who have multiple abscesses. They present with fever, toxemia, deep jaundice, and encephalopathy; toxemia is suggestive of an added bacterial infection, leading to more severe disease. Esherichia coli and Klebseilla pneumoniae are the most commonly cultured organisms, and patients are seen with a clinical picture indistinguishable from hepatic encephalopathy as a result of acute hepatocellular failure. Hepatic encephalopathy in patients with amebic liver abscess may result from a combination of right hepatic vein occlusion, pylephlebitis, and occlusion of several portal vein radicles (Kapoor & Joshi, 1992). Clinically, the usual differential diagnosis includes acute cholecystitis, hepatitis resulting from viral or other causes, and pyogenic liver abscess. With atypical presentation, hepatocellular carcinoma, a hepatic hydatid cyst, or a simple cyst may be considered (Thomas & Ravindra, 2000). Approximately three quarters of patients with an amebic liver abscess have leukocytosis. Leukocytosis most likely appears if symptoms are acute or complications have developed. Eosinophilia is rare, but mild anemia may occur in half of patients and is multifactorial. Hyperbilirubinemia is present in only a small proportion of cases.
In acute liver abscess, aspartate aminotransferase (AST) levels are high. In chronic liver abscess, the alkaline phosphatase level tends to be elevated, and the AST level tends to be within normal limits. Overall, the alkaline phosphatase level is elevated in about 70% of patients with amebic liver abscess. Similar complete blood count (CBC) and liver test abnormalities are found in patients with pyogenic liver abscesses and are not specific (Ravdin & Stauffer, 2005). Prolonged prothrombin time may occur. Chest radiography typically shows elevation of the right dome of the diaphragm with an anterior bulge on the lateral view (Debakey & Ochsner, 1951), atelectasis of the right lung, and pleural effusion. The liver shadow on plain abdominal radiographs is diffusely enlarged, and gas in the biliary tree or liver parenchyma is unusual unless there is communication of the hepatic abscess cavity with the lung or hollow viscus (Thomas & Garg, 2007).
Ultrasonography
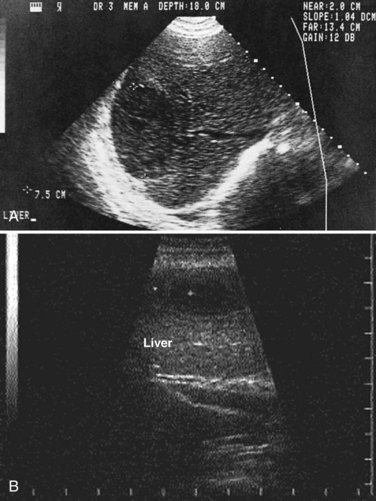
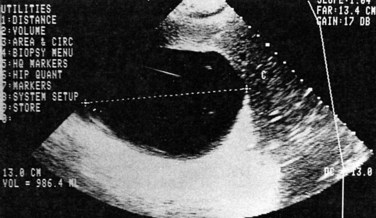
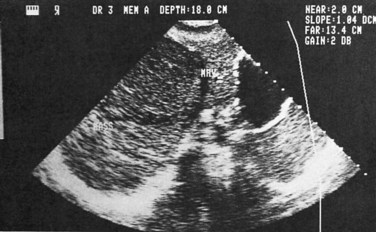
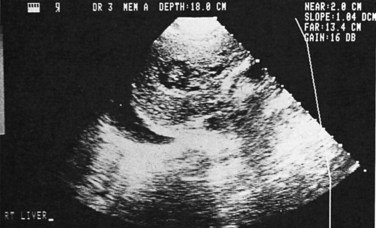
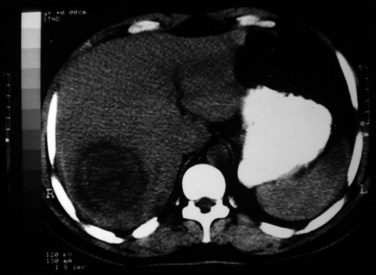
Ultrasonography (US) is the preferable diagnostic test, with an accuracy of 90% (see Chapter 13; Sepulveda & Manzo, 1986). Abscesses are usually located in contact with the liver capsule (see Fig. 67.1) and are 2 to 21 cm in size, round or oval, with well-defined margins; they are hypoechoic and clearly defined from normal liver parenchyma with distal enhancement (Figs. 67.2 to 67.4; Ralls et al, 1982; Sukougj et al, 1989). In 80% of patients, the abscess is single in the right lobe; 10% in the left lobe and 6% in the caudate lobe are single, and the remaining are multiple abscesses (Ralls et al, 1979).
In early stages, amebic abscess appears as a subtle area of decreased echogenicity, and US diagnosis is not pathognomonic; complicated cysts, hematoma, metastases, and amebic abscess may mimic each other (Elzi et al, 2004; Kapoor, 1989). Only 40% of patients have typical US features of amebic liver abscess. Serial scanning tends to show no change despite adequate treatment with amebicidal drugs, complete aspiration of the abscess, or both (Ralls et al, 1979; Sukov et al, 1980). The mean resolution time is 7 months, and complete resolution may take up to 2 years. On occasion, percutaneous diagnostic aspiration may be needed to differentiate amebic from pyogenic liver abscess (Kurland & Brann, 2004). Although pyogenic liver abscesses tend to resolve earlier (within 2 to 4 months), amebic liver abscesses acquire a more echogenic and fibrous wall in 8 to 16 weeks and begin to resemble, but must be differentiated from, an encapsulated tumor. With time, resolution may be complete, or the result may be a residual cystic cavity that resembles a simple cyst of the liver (Ralls et al, 1983; Sheen et al, 1989).
Other Imaging Modalities
Computed tomography (CT) does not add to the diagnostic accuracy of US in acute stages, except in evaluation of imminent rupture of an abscess or for detection of small lesions (see Chapter 16; Kimura et al, 1997). Amebic liver abscesses usually appear on CT with contrast as rounded, well-defined lesions with complex fluid (Radin et al, 1988). The most characteristic finding is an enhancing wall with a peripheral zone of edema around the abscess (Fig. 67.5). The abscess cavity may show multiple septa (more with pyogenic abscesses), fluid and debris levels, air bubbles, or hemorrhage. CT may detect extension of amebic liver abscesses to other organs (Radin et al, 1988).
Magnetic resonance imaging (MRI; see Chapter 17) is not superior to CT in the diagnosis of amebic liver abscess, but it may be useful in differentiating it from a hepatic neoplasm. Amebic liver abscesses appear as heterogeneous cavities that are hypointense on T1-weighted images and hyperintense on T2-weighted MR images. The abscess margin may show incomplete hyperintense rings with perilesional edema on T2-weighted images. Following treatment, the abscess cavity becomes homogeneous, and complete concentric rings appear as a result of periabscess fibrosis and hemosiderin deposits (Mortelé & Ros, 2001).
Angiography is rarely required. When performed, it reveals a hypovascular or avascular mass displacing the hepatic artery and portal vein branches but may show portal vein thrombosis (Viana, 1975). Although its role is currently limited with gallium scanning, amebic liver abscesses appear as cold spots, whereas pyogenic lesions are seen as warm spots (Kapoor, 1989).
Amebic Serology
Amebic serology is highly sensitive and specific in the differentiation between pyogenic and amebic hepatic abscess. EIA is simple, rapid, inexpensive, and more sensitive, and it has now largely replaced IHAT and counterimmunoelectrophoresis (Restrepo et al, 1996). EIA detects antibodies specific for E. histolytica in approximately 95% of patients with extraintestinal amebiasis, 70% with active intestinal infection, and 10% who are asymptomatic cyst passers. Antibody response is related to the duration of illness and may be detectable 7 to 10 days after the onset of symptoms. Titers peak by the second and third months, decreasing to lower levels by 9 months; they revert to negative by 12 months (Munoz, 1986). Monoclonal antibody-based tests allow differentiation between invasive and noninvasive parasites (Kimura et al, 1997). Enzyme-linked immunosorbent assay (ELISA) and IHAT cannot differentiate acute from remote infection in endemic areas (Thomas & Garg, 2007).
Currently, ELISA for detection of the galactose-inhibitable adherence protein in serum and feces and IHAT appear to be the most reliable tests, with sensitivity and specificity greater than 95% (Hira et al, 2001), with a reversal rate of 82% after 1 week of treatment with metronidazole (Tanyuksel & Petri, 2003). Efforts are ongoing to identify antigens specific for acute infection (Ravdin, 1995). Rapid antigen and antibody tests are being evaluated and seem very promising (Leo et al, 2006).
Role of Aspiration
US-guided aspiration (Fig. 67.6) is often justified on the basis that the diagnosis would be “more certain,” or that the abscess can be “aspirated to dryness” at the time of therapeutic aspiration. PCR may detect E. histolytica DNA in amebic liver abscess pus as well as in the saliva of patients (Khairnar & Parija, 2008; Khan et al, 2006). No randomized controlled trial has ever shown that aspiration is beneficial in survival, length of hospitalization, or time to become afebrile compared with treatment with antiamebic drugs alone (Chavez-Tapia et al, 2009; Sharma et al, 1989; Stanley, 2003; Van Allan et al, 1992), and aspiration may only confuse the diagnosis by revealing atypical pus or blood. The belief that aspiration hastens clinical recovery and may not involve significant procedure-related morbidity is widespread in clinical practice, however. This approach is supported by a small prospective study (Tandon et al, 1997) and continues to be advocated in reviews (Haque et al, 2002). Clinical improvement invariably occurs with antiamebic therapy alone in uncomplicated cases. When the differential diagnosis in a given case includes operable neoplasm or hydatid disease, aspiration is risky and may be contraindicated (Thomas & Garg, 2007).
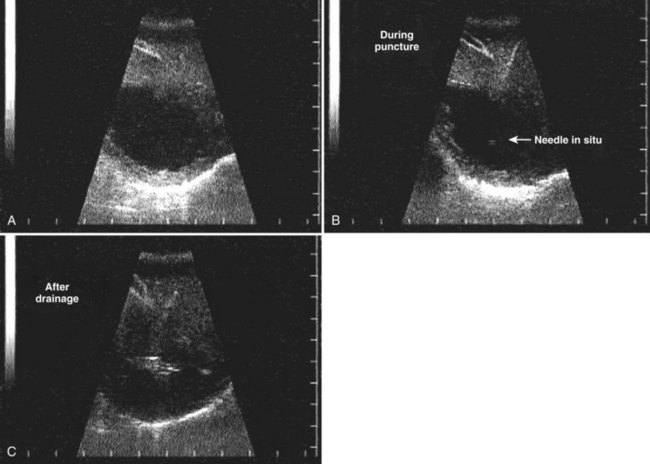
FIGURE 67.6 Ultrasound showing a typical liver abscess. A, Before drainage. B, During drainage. C, After drainage.
(Courtesy Professor A.K. El Dory, Ain Shams University, Cairo.)
Therapeutic aspiration should be reserved for the following situations (Ralls et al, 1982):
1 Serology is inconclusive and differential diagnosis is pyogenic liver abscess.
2 A therapeutic trial with antiamebic drugs is deemed inappropriate, as in pregnancy.
3 The liver abscess is secondarily infected, estimated to be true in 15% of cases (McDermott, 1995).
4 Fever and pain persist for more than 5 to 7 days after starting appropriate therapy.
5 Rupture is imminent in an extremely large abscess (>10 cm), especially if pericardial rupture from a left lobe abscess appears likely.
The following factors are predictive of the need for aspiration: 1) age 55 years or older, 2) an abscess 5 cm or more in diameter, and 3) failure of medical therapy after 7 days (Khan et al, 2008). In endemic areas, because of late presentation and existence of multiple abscesses, up to 50% of patients may require aspiration (Khanna et al, 2005). A single aspiration may be sufficient for diagnostic purposes, but it is inaccurate to recommend it because the characteristic “anchovy paste” characteristic of amebic abscess may not be found; when performed as part of therapy, it will likely be inadequate. Percutaneous catheter drainage (PCD) is better than percutaneous needle aspiration (PNA) for management of large liver abscesses (>10 cm) in terms of duration to attain clinical relief and duration for which parenteral antibiotics are needed (Singh et al, 2009).
Complications
Peritoneal and Visceral Involvement
Peritonitis with amebiasis is due to rupture of an amebic liver abscess in 78% of patients and perforated or necrotizing amebic colitis in the other 22% (Ken et al, 1989; Monga et al, 1976). Spontaneous rupture of amebic liver abscess may occur in 2.7% to 17% of cases (Eggleston et al, 1982; Monga et al, 1976; Sarda et al, 1989). Adherence of the liver abscess to the diaphragm, abdominal wall, omentum, or bowel tends to confine contamination and lead to rupture into hollow viscera, such as the stomach or colon (Angel et al, 2000).
A hepatogastric, hepatoduodenal, or hepatocolonic fistula and acute hepatic failure may occur. Free rupture into the peritoneal cavity is uncommon and usually only occurs in a nutritionally depleted patient (Rao et al, 2009). Patients present with abdominal pain, a palpable mass, or generalized distension. Bloody diarrhea may occur in colonic rupture, and hematemesis may occur in cases of hepatogastric fistula. Signs of peritonitis with tender hepatomegaly, intercostal tenderness, right basal lung signs, and clinical jaundice may lead to suspicion of the diagnosis, which can be confirmed on US. The diagnosis may be made only at laparotomy, with risk of bleeding from decreased prothrombin levels (Thomas & Garg, 2007). US and CT often show a perihepatic fluid collection in cases of amebic liver abscess that are reactive or with actual leaks from the abscess cavity (Radin et al, 1988). Management of abscesses that extended into the peritoneal cavity with aggressive surgical approaches were associated with increased mortality rates (Balasegaram, 1981; Eggleston et al, 1982) and have now been replaced by increasingly successful attempts at percutaneous drainage of the liver abscess and the extravasated pus (Ken et al, 1989; Sarda et al, 1989).
Absolute indications for laparotomy include a doubtful diagnosis; concomitant hollow viscus perforation with fistulization, resulting in life-threatening hemorrhage or sepsis; and failure of conservative management. At laparotomy, the liver abscess, which usually appears as a tan-colored bulge on the surface, must be handled gently. Septa running across the cavity are usually blood vessels and bile ducts traversing the abscess cavity. Hemorrhage can be difficult to control, and postoperative bile leaks may result. Endoscopic stenting or nasobiliary drainage may be required in cases of biliary communication (Sandeep et al, 2006). Irrigation of the abscess cavity with saline is usually sufficient and may be followed by the installation for 3 to 5 minutes of a solution of 65 mg of emetine hydrochloride in 100 mL of normal saline. Tube drains are inserted and retained as necessary. Hollow viscus perforations must be dealt with on an individual basis, with exteriorization, proximal diversion, or serosal patch closure (Thomas & Garg, 2007). Postoperative intravenous metronidazole is combined with broad-spectrum antibiotics, and dehydroemetine is added if no cardiac contraindication exists. The mortality rate of viscus perforation ranges from 12% to 50% (Sarda et al, 1989).
Thoracic and Pleuropulmonary Involvement
Pulmonary complications occur in 7% to 20% of patients with amebic liver abscess (Stanley, 2003). A sympathetic right-sided effusion is the most common pulmonary complication and usually does not require treatment itself. Other thoracic complications include rupture of the abscess into the pleural cavity or into the bronchial tree. This condition manifests as dyspnea and dry cough with right basal crepitations and collapse of the right lung in addition to the abdominal signs. A pleural rub may also be found. Sudden onset of coughing with expectoration of copious quantities of chocolate-colored sputum occurs if the abscess ruptures into bronchi (Guarner, 1986). There are several routes of pulmonary infection, including direct hematogenous and inhalational amebiasis (Shamsuzzaman & Hashiguchi, 2002). Thoracocentesis is the main line of treatment. In case of aspiration and intercostal tube drainage, care is taken to go high on the right lateral side of the chest near the axilla. Ineffective early drainage of the amebic empyema is usually complicated by secondary infection that requires aggressive surgical procedures, such as pulmonary decortication, because the fibrous tissue can be dense enough to complicate thoracoscopic surgical techniques (Thomas & Garg, 2007).
Lung abscess rarely occurs, and it is walled off from the pleural and peritoneal cavities; surgical intervention is not required because postural drainage, bronchodilators, and antiamebic drugs may suffice. Metronidazole is effective, but emetine produces a rapid response and may be required in cases of metronidazole resistance (Jain et al, 1990).
Vascular and Pericardial Involvement
Some cases of vascular complications have been reported, such as Budd-Chiari syndrome (Mechai et al, 2009) and right atrial and inferior vena cava thrombosis, complicating multiple large amebic liver abscesses (Khan & Ameen, 2009; Sodhi et al, 2009). Surgical removal of the thrombus may be required.
Abscess rupture into pericardium is rare but serious, occurring in 1.3% to 2% of patients, with mortality rates from 30% to 60% (Shamsuzzaman & Hashiguchi, 2002). Abscesses of the left lobe of the liver or those more centrally located are more prone to pericardial complications that range from asymptomatic pericardial effusion to cardiac tamponade. Although left lobe abscesses resolve equally well with antiamebic drugs, as do right-sided abscesses (Thompson et al, 1985), the detection of pericardial thickening or pericardial effusion may constitute an indication for aspiration of a left-sided amebic liver abscess (Radin et al, 1988). In cardiac tamponade, pericardiocentesis must be performed, along with drainage of the liver abscess followed by antiamebic drugs, namely metronidazole. Dehydroemetine is used with caution because of its cardiotoxicity (Thomas & Garg, 2007).
Chemotherapy
Metronidazole
Metronidazole is the drug of choice for amebic liver abscess. The oral dose is 500 to 750 mg three times daily for 7 to 10 days, the same dose and duration used for intestinal amebiasis in adults, and 35 to 50 mg/kg in three divided doses for 10 days in children, with a cure rate of more than 90% (Li & Stanley, 1996). Hepatopulmonary amebiasis was found to respond equally to doses of 400 and 800 mg three times daily given over 5 days (Jain et al, 1990). The intravenous route is also highly effective at a recommended dose of 500 mg every 6 hours. Metronidazole reaches high concentrations in the liver and intestine, and it crosses the placenta and the blood-brain barrier. Its use is contraindicated in the first trimester of pregnancy and must be used cautiously in the second and third trimesters; breastfeeding should be discontinued during its use. The response of patients with amebic liver abscess to metronidazole is profound, with improvement in symptoms within 72 to 96 hours. However, a luminal agent such as paromomycin (30 mg/kg three times a day for 5 to 7 days), iodoquinol (650 mg orally three times a day for 20 days), or diloxanide furoate (500 mg orally three times a day for 10 days) should also be used to eradicate intestinal colonization (Stanley, 2003).
Medical therapy has a disulfiram-like action. The most common side effects are nausea, a metallic taste in the mouth, vomiting, and dark brown discoloration of the urine. Over 5 days, an 85% cure rate is achieved, which may increase to 95% after 10 days (Guarner, 1986). From 5% to 15% of patients with amebic liver abscess may be resistant to metronidazole (Thompson et al, 1985), but this may not be a major clinical problem (Li & Stanley, 1996); most reports of “drug resistance” reflect delayed resolution of either clinical symptoms or US findings and not a true resistance documented by drug failure. Experimental resistance may be related to inducing superoxide dismutase in vitro (Wells & Arguedas, 2004). Alternate medical therapy, percutaneous aspiration, or surgical intervention usually must be considered in patients who do not respond or show only modest improvement in 72 hours (Thompson et al, 1985). In countries where they are available, tinidazole, ornidazole, and nitazoxanide are alternative agents for the treatment of amebic liver abscess; these drugs are administered for only a few days (Lasserre et al, 1983; Quaderi et al, 1978; Vanijanonta et al, 1985). One study of a single 2-g dose of either tinidazole or ornidazole gave a success rate of 94% in both treatment arms (Lasserre et al, 1983). Other studies have shown a success rate of almost 100% for patients treated with tinidazole for 2 to 3 days (Khokhani et al, 1978; Quaderi et al, 1978; Vanijanonta et al, 1985). Tinidazole has been approved by the Food and Drug Administration (FDA) for the treatment of amebiasis, including amebic liver abscess. Secnidazole has a longer half-life, and a single daily dose of 2 g for 5 days is effective (Salles et al, 2003). Satranidazole showed lower incidence of side effects and better tolerance than metronidazole in a randomized, single-blind trial of 49 patients with amebic liver abscess (Muzaffar et al, 2006).
Other Medications
Emetine hydrochloride is effective against trophozoites and reaches amebicidal concentrations in tissues rather than intestine. Emetine is administered by intramuscular or deep subcutaneous injection in a dose of 1 mg/kg/day (maximum 60 mg/day) for 10 days. The patient must be placed on complete bed rest. Tissue levels persist for 40 to 60 days, and readministration should be avoided for 6 weeks. Side effects include myositis at the injection site, hypotension, tachycardia, chest pain, dyspnea, and abnormalities on electrocardiogram, including T-wave inversion and prolonged Q-T interval. The drug is contraindicated in renal, cardiac, and muscular disease and is used cautiously in children and elderly patients (Thomas & Garg, 2007). Emetine or dehydroemetine is valuable in treatment of hepatopulmonary amebiasis (Guarner, 1986; Jain et al, 1990; Thompson et al, 1985).
Chloroquine phosphate is antimalarial and is effective in patients with resistance to emetine and pulmonary amebiasis (Guarner, 1986) but has no luminal amebicidal activity. Chloroquine is administered orally in a dose of 1 g (600 mg base) per day for 2 days followed by 500 mg (300 mg base) per day for 2 to 3 weeks. It is contraindicated in patients with retinopathy, but it has been used in pregnant patients (Guarner, 1986). Diloxanide is ineffective in invasive amebiasis and is used for treatment of asymptomatic carriers. The recommended dose is 500 mg three times a day for 10 days. No serious side effects are reported.
Nitazoxanide was effective in treating invasive amebiasis and eliminating colonization in intestine. Further studies are warranted in hepatic amebiasis (Rossignol et al, 2007). Trifluoromethionine is a lead compound, but a single subcutaneous or oral dose prevented the formation of amebic liver abscess in a rodent model (Sato et al, 2010).
Therapeutic Strategy
Oral metronidazole is administered as a single drug, with concomitant correction of hypoprothrombinemia, hypoproteinemia, and anemia. If dramatic improvement occurs in 48 to 72 hours, no therapy other than the complete course of metronidazole is required. A luminal agent, such as diloxanide furoate (500 mg three times a day for 10 days) or paromomycin (30 mg/kg/day in three doses for 10 days), must be administered after metronidazole therapy for eradication of intestinal infection as part of a complete treatment regimen (Irusen et al, 1992).
In patients who do not respond satisfactorily, emetine or dehydroemetine is added. Evidence of pulmonary, peritoneal, or pericardial extension is an indication for aspiration of the liver abscess with an intercostal tube or catheter drainage. Percutaneous catheter drainage is usually recommended for abscesses larger than 10 cm in diameter and for left lobe liver abscess, failure of medical treatment for 7 days, and failure to differentiate the abscess from a pyogenic abscess (Singh et al, 2009). The following are predictive of the need for aspiration: 1) age older than 55 years, 2) abscess greater than 10 cm in diameter, and 3) failure of medical therapy after 7 days (Khan et al, 2008). Late presentation with the existence of multiple abscesses may require aspiration (Khanna et al, 2005), but prompt medical care decreases the need. Laparotomy is usually reserved for patients with suspected peritonitis, fistulization, or secondary infection with sepsis after failure of above measures.
A meta-analysis of patients with amebic liver abscess showed that 4% died (Pitt, 1990). In patients treated with amebicidal drugs alone, the mortality rate was 2%. Independent risk factors for death include serum bilirubin greater than 3.5 mg/dL; encephalopathy; hypoalbuminemia, defined as less than 2 mg/dL; and multiple abscess cavities (Sharma et al, 1996) or total abscess volume greater than 500 mL (Wells & Arguedas, 2004). Rupture into the peritoneal cavity and the pericardium is responsible for most deaths. Patients treated with early and aggressive surgery as advocated by some authors (Balasegaram, 1981; Eggleston et al, 1982
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree