Chapter 88 Advances in systemic therapy for hepatocellular carcinoma
Overview
Hepatocellular carcinoma (HCC) is the fifth most common solid tumor and the third most common cause of cancer death worldwide (Abou-Alfa, 2008; see Chapter 80). Its incidence mirrors that of chronic liver injury, most commonly the result of viral infection by both hepatitis B and C (see Chapter 64). Other etiologies that lead to chronic liver injury and cirrhosis include alcohol abuse; nonalcoholic steatohepatitis, commonly associated with morbid obesity and diabetes; and other metabolic diseases, such as hemochromatosis. The highest incidence of HCC remains in Southeast Asia and sub-Saharan Africa (McGlynn et al, 2001); however, concern continues about the rising incidence of HCC in North America (El-Serag & Mason, 1999). A threefold increase in the age-adjusted rates for HCC associated with hepatitis C virus (HCV) infection has been observed, from 2.3 per 100,000 between 1993 and 1995 to 7.0 per 100,000 between 1996 and 1998; this is most likely explained by the increased incidence of HCV in North America during that period.
Hepatocellular Carcinoma and Cirrhosis: two Diseases in One (See Chapter 70A, Chapter 70B )
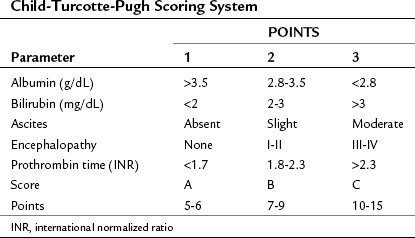
Both cirrhosis and the tumor itself impact the HCC patient’s overall survival (OS), thus the cirrhosis status of patients with liver cancer must be evaluated. A scoring system was originally developed by Child (1964) that consisted of three parameters: jaundice (bilirubin), ascites, and encephalopathy. This scoring system was later updated by Pugh et al (1973), who added an assessment of hepatic synthetic function by evaluating serum albumin levels and prothrombin time (PT) (Table 88.1; see Chapters 2 and 70B). This combined score is known as the Child-Turcotte-Pugh (CTP) score, and it remains the most commonly used scoring system for liver cirrhosis. The pitfall of this classification is its lack of any parameters that relate to the cancer itself, as it was developed in patients who have cirrhosis but no cancer. As such, it is unlikely to be the best predictor of outcome in patients with HCC.
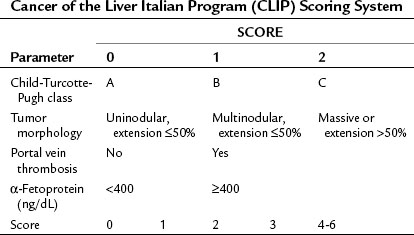
Okuda and colleagues (1985) recognized the need for an HCC staging system that incorporated factors related to both the cirrhosis and the tumor itself and developed what is known as the Okuda staging system, which encompasses four variables: 1) albumin, 2) bilirubin, 3) ascites, and 4) tumor size as a percentage of the size of the liver (greater or less than 50%). The Okuda staging system served as a platform for more advanced and sophisticated prospective scoring systems that used the Cox proportional hazard regression model. The Cancer of the Liver Italian Program (CLIP) developed a scoring system in patients with HCC, with mainly HCV as an etiology (CLIP, 1998, 2000), that found the independent prognostic variables for patients with HCC to be the CTP score plus three additional variables that relate to the tumor itself: tumor morphology, as assessed by the number of lesions and extent of disease in the liver; presence or absence of portal vein thrombosis; and α-fetoprotein (AFP) level (Table 88.2). Patients with a high score (4 to 6) were shown to have a median survival of only 3.2 months.
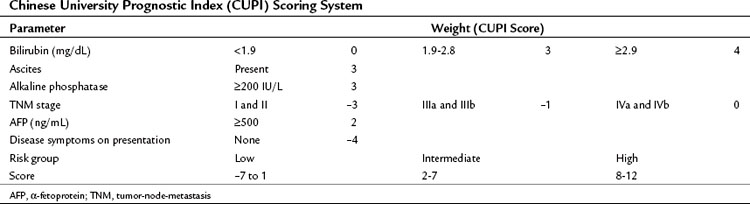
In contrast to the CLIP scoring system, the Chinese University Prognostic Index (CUPI), another scoring system based on multivariate analysis, was developed mainly in patients with hepatitis B virus (HBV)-associated HCC (Leung et al, 2002). This prognostic index identified bilirubin, alkaline phosphatase, and the presence or absence of ascites, in addition to tumor stage based on the tumor-node-metastasis (TNM) staging and AFP levels, as the most important predictors of outcome. In addition, a clinical assessment parameter of presence or absence of symptoms at presentation was incorporated (Table 88.3). An important aspect of the CUPI index is that different weights are attributed to the different parameters. The median survival of the highest risk group was close to 1 month. Other scoring systems include the Groupe d’Étude et de Traitement du Carcinome Hépatocellulaire (GETCH) staging system (Chevret et al, 1999), the Japan Integrated Staging (JIS) Score (Kudo et al, 2003), and the Barcelona Clinic Liver Cancer (BCLC) classification system (Llovet et al, 1999).
In a retrospective analysis of patients with advanced HCC seen by medical oncologists at Memorial Sloan-Kettering Cancer Center (MSKCC) over a period of 5 years, we attempted to identify which of these eight scoring systems would be most valuable in this specific clinical setting (Huitzil-Melendez et al, 2010). By using a concordance index, the GETCH, CLIP, and CUPI were found to be the most informative staging systems in predicting survival in patients with advanced HCC. The BCLC system did not score well in this exercise, which studied a specific niche of patients with advanced HCC, all of whom fell within the single basket of category C of BCLC; therefore it lacks any discriminatory value. Other groups have independently come to the same conclusion regarding the BCLC system (Collette et al, 2007).
Role of Chemotherapy in Hepatocellular Carcinoma
Almost all classes of chemotherapy have been studied in advanced HCC (Nerenstone et al, 1988). Doxorubicin is one of the most studied drugs in this disease, partly as a result of what has thus far been an irreproducible response rate of 79%, originally reported by Olweny and colleagues (1975). It should be noted that this early report from the mid-1970s did not use modern computerized tomography (CT) or magnetic resonance imaging (MRI) methods available and used routinely today. The majority of “responses” were determined either on the basis of physical examinations of the liver or using a colloidal gold liver scan, measuring techniques that would not be acceptable as evidence of a response in clinical trials by current standards. Many subsequent attempts to try to reproduce this high response rate have been made, both with doxorubicin as a single agent (Barbare et al, 1984; Chlebowski et al, 1984; Ihde et al, 1977; Johnson et al, 1978; Sciarrino et al, 1985; Vogel, 1977; Williams & Melia, 1980) and in combination with other chemotherapeutic agents (Choi et al, 1984; Falkson et al, 1978, 1984; Melia et al, 1983; Olweny et al, 1980), but to no avail.
Other older and newer chemotherapeutic agents have also been studied in HCC, including cisplatin (Falkson et al, 1987), etoposide (Melia et al, 1983), mitoxantrone (Falkson et al, 1987), vinblastine (Damrongsak et al, 1973), capecitabine (Patt et al, 2004), gemcitabine (Kubicka et al, 2001), irinotecan (O’Reilly et al, 2001), and paclitaxel (Chao et al, 1998), all with reported dismal response rates and no impact on survival.
Although several combination chemotherapy agents have shown improved response rates, the impact on survival was not noticeable in most cases (Baker et al, 1977; Bobbio-Pallavicini et al, 1997; Porta et al, 1995). As a single agent, interferon α-2b in a trial by the Gastrointestinal Tumor Study Group (GITSG, 1990) showed a limited response rate of only 7%. This disappointingly low response, in addition to increased toxicity as a result of high doses of interferon, led to testing lower doses in combination with chemotherapy. Despite a reported high response rate of 31% in some trials (Ji et al, 1996; Patt et al, 1993), favorable results have not been reproducible (Kardinal et al, 1993; Stuart et al, 1996).
Nevertheless, the preliminary indication of some activity for interferon set the platform for a more intense combination of cisplatin, interferon, doxorubicin, and 5-fluorouracil (5-FU), which became commonly known as PIAF (Patt et al, 1999). PIAF was subsequently modified and tested in the outpatient setting (Leung et al, 1999), and a Phase II trial of 50 patients showed a response rate of 26%. More importantly, nine patients (18%) had their tumor resected after therapy, and of these resected patients, four had a pathologic complete response noted in the resected tumor. Considerable hematologic toxicity was reported in this 50-patient cohort, and two treatment-related deaths resulted from neutropenic fever. It is important to note that this study was conducted before granulocyte-stimulating factors were commonly used for similar intense chemotherapeutic regimens.
These data led to a randomized Phase III trial of doxorubicin versus a combination, PIAF (Yeo et al, 2005). This trial showed the same 21% response rate for PIAF versus 10% for single-agent doxorubicin; however, the study failed to show any survival advantage in favor of the PIAF combination (8.7 vs. 6.8 months; P = .83). Despite this trial having a negative outcome for the primary end point, it did provide several important and useful pieces of information: First, as noted above, it identified what can realistically be considered the true response rate—by current standards of response definition and determination—of doxorubicin in HCC, and that response rate is 10%. Furthermore, although the study failed to provide support for use of PIAF as a routine palliative therapy for advanced disease, it might be considered as a conversion therapy in very carefully selected patients with potentially resectable tumors.
Some of the generally disappointing results are explained by the genetic makeup of HCC, which comprises highly resistant clones of cancer cells (DeVita & Abou-Alfa, 2000). The HCC cells carry a high genetic mutation load, which makes them less amenable to the destructive actions of chemotherapy. HCC cells usually contain a high level of dihydropyrimidine dehydrogenase (DPD), which potentially makes them relatively resistant to 5-FU (Jiang, 1993). HCC cells also overexpress the multidrug resistance gene MDR1 (Chenivesse, 1997) and the gene product P-glycoprotein (P-gp) (Soini et al, 1996). This may help explain the resistance of HCC to paclitaxel (Chao et al, 1998), but it would not necessarily explain the PIAF pathologic response (Leung et al, 1999), the witness to the efficacy that chemotherapy can exert onto HCC, contrary to all attestations that it cannot; this concept will be revisited as part of its evaluation in combination with biologic therapy (Abou-Alfa, 2008).
Several attempts at overcoming this resistance by developing novel cytotoxic agents have been made, although not with any appreciable success. T138067 is an antimicrotubular non–P-gp substrate (Shan et al, 1999; Venook et al, 2004) that showed a modest 9% response in chemotherapy naïve patients (Leung et al, 2002); however, a randomized Phase II/III trial of T138067 versus doxorubicin in patients with advanced HCC was prematurely closed because of lack of survival benefit (Posey et al, 2005). Nolatrexed dihydrochloride is a nonclassic lipophilic inhibitor of thymidylate synthase that is not catabolized by dihydropyrimidine dehydrogenase. It lacks a glutamate side chain and thus has reduced potential transport-associated resistance (Webber et al, 1993). Nolatrexed was studied in two different clinical trials; however, results were disappointing, with an 8% response rate in a Phase II North American study (Stuart, 1999) and no responses in a randomized Phase II study in Hong Kong (Mok et al, 1999). Despite those disappointing results, a randomized Phase III trial that evaluated nolatrexed versus doxorubicin in patients with unresectable HCC was performed. It showed a median OS of 5.6 months for nolatrexed versus 8 months for doxorubicin (P = .0068) (Gish et al, 2007). It is unclear whether nolatrexed conferred a negative survival effect or was simply inactive.
A novel modality of administering floxuridine (FUDR) and dexamethasone via hepatic arterial infusion (HAI) was also studied in HCC (Jarnagin et al, 2009). Among eight patients studied, the response rate was 25%, and the hepatic progression-free survival (PFS) was 9.4 months. Interestingly, patients in the same study with cholangiocarcinoma fared better, with a 53.8% response rate and 11.6 months hepatic PFS. HAI is discussed in detail in Chapter 86.
Novel Biologic Therapies in Hepatocellular Carcinoma
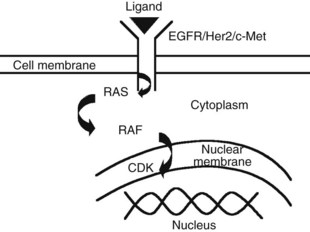
The advent of novel targeted therapeutics and the lack of a consistent standard of care for advanced HCC has led to interest in identifying and evaluating relevant targets in HCC along the signal transduction pathways (Huitzil-Melendez et al, 2009; Fig. 88.1).
Epidermal Growth Factor Receptor, c-MET, and Insulin Growth Factor Receptor
The epidermal growth factor receptor (EGFR) is one of the most thoroughly studied therapeutic targets. Several authors have reported no difference in expression of EGFR between HCC and noncancerous diseased liver tissues (Harada et al, 1999; Kira et al, 1997), whereas others have reported overexpression in 17% of HCC cases (Kiss et al, 1997). Erlotinib, an EGFR-specific receptor tyrosine kinase inhibitor, was tested in HCC as part of a Phase II trial of 38 patients with advanced HCC (Philip et al, 2005). Close to half of the patients had prior therapy, and 71% of all patients had class A cirrhosis. The primary end point was PFS at 6 months, using Response Evaluation Criteria in Solid Tumors (RECIST), and this end point was reached in 12 (32%) of 38 patients, with a median PFS of 3.8 months. In addition, three partial responses (8%) were reported, and median OS was 13 months. Similar to the experience with other tumors, immunohistochemical staining of EFGR expression was not associated with outcome. The most frequent grade 3 and 4 toxicities were skin rash (13%), diarrhea (8%), and fatigue (8%).
Despite having some preclinical activity in HCC (Huether et al, 2005), cetuximab has not been tested in HCC. Lapatinib, an oral agent with dual inhibition of EGFR and tyrosine kinase 1 and 2 (Her2/Neu), has been studied in HCC (Ramanathan et al, 2009) despite the rare expression of Her2/Neu in human HCC tissues (Hsu et al, 2002) and with disappointing results. In a Phase II trial, 40 patients with advanced HCC were treated with lapatinib, and the response rate was 5%, PFS was 2.3 months (95% confidence interval [CI], 1.7 to 5.6), and median survival was 6.2 months. Both fall short of other reported data in the literature, and EGFR genotyping indicates that HCC patients with less than 20 repeats have the worst PFS.
Another potential target is the hepatocyte growth factor (HGF) and its receptor c-Met, which were found to be overexpressed in 33% and 20% of human HCC tissues, respectively (Kiss et al, 1997). In another study, c-Met was found to be overexpressed preferentially in early stage resected HCC, with no association with outcome as measured by OS (Huitzil-Melendez et al, 2009). Certain c-Met inhibitors are already being studied in HCC, such as ARQ 197, which was already evaluated in a Phase I study (Garcia et al, 2007).
Sorafenib
Sorafenib is a novel molecular targeted agent that inhibits the serine/threonine kinase Raf-1 in vitro in addition to the proangiogenic (vascular endothelial growth factor receptor [VEGFR]-1, -2, and -3; platelet-derived growth factor receptor [PDGFR]-β); and tumorigenic receptor tyrosine kinases (RTKs; RET, Flt-3, and c-Kit) (Wilhelm et al, 2004). Sorafenib has been studied extensively in HCC and has now been approved by regulatory agencies worldwide as a standard therapy for unresectable HCC.
HCC is a highly vascular solid tumor with high expression of VEGF, which plays a major role in HCC development and potentiates its metastatic capability in preclinical models (Yoshiji et al, 2004). Proangiogenic platelet-derived growth factor receptor-β (PDGFR-β) also contributes to this increased metastatic potential (Zhang et al, 2005).
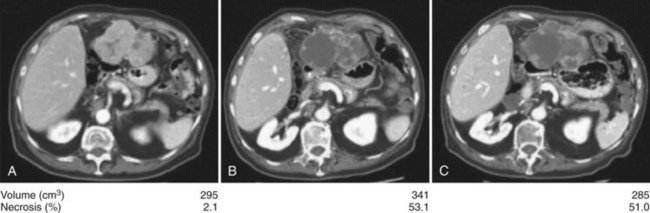
Anti–VEGFR-1, -2, and -3 and PDGFR-β sorafenib are the most studied antiangiogenic drugs in HCC so far; although an initial Phase II trial evaluating response to sorafenib in patients with advanced HCC showed no significant response rate (2%) (Abou-Alfa et al, 2006), 34% of patients had stable disease of a minimum 4 months’ duration. This was commensurate with a median time to progression (TTP) of 4.2 months and median OS of 9.2 months, both of which compare favorably to historic controls (Yeo et al, 2005). The main grade 3 and 4 toxicities were fatigue (9.5%), diarrhea (8%), and hand-foot skin reaction (5.1%), known also as hand-foot syndrome. The high rate of stable disease was associated with an observed phenomenon of central tumor necrosis in many patients in the study whose tumors were evaluated using triphasic CT scans inclusive of an arterial phase (Fig. 88.2). This central tumor necrosis was quantified, and the volume was assessed using a computer algorithm for semiautomated delineation of tumors (Zhao et al, 2006). It was later found that the ratio of the percentage of the described tumor necrosis over the tumor volume correlated with objective response (Abou-Alfa, 2008). This phenomenon is still pending validation and is currently the subject of many prospective correlative studies of ongoing HCC clinical trials.
The suggested improved outcome noted in this Phase II study led to a large, double-blinded, randomized Phase III trial evaluating sorafenib versus placebo in patients with advanced HCC and class A cirrhosis (Llovet et al, 2008) with two primary end points of OS and time to symptomatic progression (TTSP) using the FHSI8-TSP instrument. This Phase III study, known for short as the SHARP trial (Sorafenib HCC Assessment Randomized Protocol), demonstrated a survival of 10.7 months in the sorafenib group versus 7.9 months in the placebo group (P < .001, hazard ratio [HR], 0.69). The second primary end point in evaluating TTSP showed no difference between the two arms (P = .77). This observation is limited by the poor understanding of the validity of the FHS18-TSP instrument in this setting. Add to the fact that a large number of the patients in this study—including 17% with locally advanced tumor, BCLC B patients—had an excellent performance status and lacked any symptoms that the FHS18-TSP instrument would otherwise depend on for its measurements. The toxicity profile was similar to that noted in the Phase II study, with 8% grade 3 to 4 diarrhea and hand-foot syndrome. Rare bleeding events were observed (<1%) that would still force a word of caution considering the antiangiogenic nature of sorafenib, which is true also for other agents in its class, such as bevacizumab and sunitinib, that may cause fatal hemorrhage (Siegel et al, 2008; Faivre et al, 2009).
A second randomized Phase III study to evaluate sorafenib in patients with advanced HCC and class A cirrhosis was conducted in the Asia-Pacific geographical area (Cheng et al, 2009). The study had eligibility criteria similar to the SHARP trial but had two differences in the design: 1) the study had a 2 : 1 randomization, typically to help encourage accrual, and 2) it did not have a predefined primary end point but rather looked at several. Similar to the SHARP trial, the Asia-Pacific study showed an improvement in survival in favor of sorafenib (6.5 months) versus placebo (4.2 months); however, this statistically significant improvement (P = .014) was not of the same magnitude as that of the SHARP trial, despite similar HRs—0.68 and 0.69, respectively—in the Asia-Pacific and SHARP studies. In an attempt to explain the difference in magnitude of the OS, it was argued that in the Asia-Pacific study, patients were more ill at the time of accrual (Abou-Alfa et al, 2009) and had more extensive disease at a more advanced stage. This observation may partly explain the difference in magnitude of benefit from sorafenib between those two populations, and it may suggest, in view of the similar HRs, that the benefit for sorafenib was expressed on the survival curve at two different times in the natural history of the disease: earlier in the SHARP trial and later in the Asia-Pacific study.
In a subgroup analysis of the SHARP trial, it was noted that sorafenib-treated patients with HCV-based HCC (n = 93) had a median survival advantage of 14 months compared with the entire sorafenib-treated group, whose survival was only 10.7 months (Bolondi, 2008); this suggests a possible positive influence of HCV status on the efficacy of sorafenib. The placebo-controlled HCV group did not have any added survival advantage over the placebo population of the study, thus proving the lack of any advantage brought on by the HCV itself. Of note, in HCV infection, the virus core proteins result in high basal activity of Raf-1, which leads to a sustained response to EGFR by hepatocytes and results in an increased possibility of neoplastic transformation (Giambartolomei et al, 2001). A similar observation was noted in a retrospective analysis of the Phase II trial that evaluated sorafenib in patients with advanced HCC (Abou-Alfa et al, 2006). In this analysis, it was noted that patients who were infected with HCV but not HBV (n = 13) had a longer time to progression of 6.5 months, compared with 4 months (P = .05) for the patients infected with HBV (n = 33; Huitzil et al, 2008). A similar trend was noted in survival advantage (P = .29) for the HCV patients (12.4 months) versus HBV patients (7.3 months), with 73% of those accrued in the Asia-Pacific study found to have HBV as an underlying etiology for their HCC, versus only 18% of patients in the SHARP trial; this may offer another explanation, or at least a complementary one, for this difference in the outcome magnitude between the two studies. Of note, the outcome of the 18% of patients with HBV in the SHARP trial for unspecified reasons is still not reported; however, this observation does undermine the antiangiogenic effect of sorafenib, which continues to be indicated for all patients with unresectable HCC irrespective of the etiology of their cancer.
Patients with unresectable HCC and CTP class A cirrhosis who are eligible for sorafenib based on the SHARP trial encompass no more than half of the patients seen by medical oncologists (Huitzil, 2010). The safety and efficacy of sorafenib in patients with class B or C cirrhosis, who make up the other half, remain a subject of discussion. In the Phase II study that evaluated sorafenib in HCC (Abou-Alfa et al, 2006), 28% of patients had class B cirrhosis and pharmacokinetic profiles that included area under the curve (AUC) and maximun drug concentration (Cmax) comparable to patients with class A disease. The class B patients had more frequent worsening of liver function, including elevated total serum bilirubin, worsening ascites, and encephalopathy (Abou-Alfa, 2008
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree