, Anja Landowski1 and Mahesh Jayanna1
(1)
Lyell McEwin Hospital, Haydown Road, Elizabeth Vale, 5112, SA, Australia
(2)
University of Adelaide, Adelaide, SA, Australia
Keywords
Advanced endoscopic imagingEnhanced imagingLower gastrointestinal tractKudo’s pit patternEarly colorectal cancerColorectal lesion assessmentIntroduction
Gastrointestinal cancers are often preceded by a curable, non-invasive, premalignant stage that may progress asymptomatically. The transformation process begins with the formation of atypical cells in the epithelium, directly above the basal membrane. Early screening may enable detection of anomalies at very early stages before neoplasia permeates into the deeper submucosal layer and beyond. Morphological characteristics of the mucosa can then be interrogated to obtain further information, which may aid the endoscopist in deciding whether to proceed with endoscopic biopsies or resection.
Detection
Most newer electronic image enhancing modalities perform no better than white light endoscopy in the detection of colorectal neoplasia (Table 6.1). There is simply no substitute for good bowel preparation and a meticulous withdrawal technique, ensuring clear views are maintained to enable careful interrogation of mucosal folds.
Table 6.1
Image enhanced endoscopy in detecting colorectal polyp
Author | Year | Technology | # of pts | Design | Result |
|---|---|---|---|---|---|
Rex [1] | 2007 | NBI | 434 | RCT | − |
Inoue [2] | 2008 | NBI | 243 | RCT | − |
Adler [3] | 2008 | NBI | 401 | RCT | − |
Matsuda [4] | 2008 | AFI | 167 | Random, Tandem | + |
Pohl [5] | 2008 | FICE | 764 | RCT | − |
Kaltenbach [6] | 2008 | NBI | 276 | Random, Tandem | − |
Van Den Broek [7] | 2009 | AFI | 100 | Random, Tandem | − |
Adler [8] | 2009 | NBI | 1,256 | RCT | − |
Characterization
Lesions which are detected should be interrogated further to gain additional information should be studied further to gain valuable information. Ideally, this is performed in a methodical manner, initially with a “wide field” overview of the lesion where the Paris classification and granularity are assessed, followed by a closer or “micro” or “magnified” view where the vascular patterns are visualised, with some of the electronic chromoendoscopy techniques. If further information is needed and where possible, the Kudo’s pit pattern is assessed. In vivo classification of polyps using advanced imaging is important for two reasons: (1) the significant cost of histological examination of all polyps, particularly small low-risk lesions, has prompted consideration of a “diagnose, resect, and discard” strategy which could be cost effective, and (2) accurately differentiating invasive from non-invasive cancers may enable clinical decisions to be made in real time, thereby immediately guiding therapy.
Lesion Assessment with Wide Field View
Paris Japanese Classification
The Paris Japanese classification system is especially important not only for standardization but also because it allows prediction of submucosal invasion risk [9].
Polyps can be divided into:
1.
Protruding lesions (Fig. 6.1)
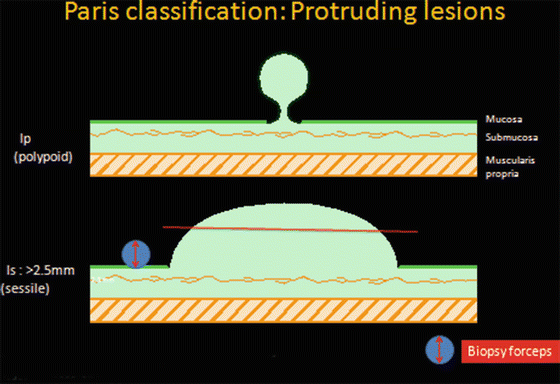

Fig. 6.1
Paris Japanese classification for protruding lesion
(a)
Ip (peduncalated)
(b)
Is (sessile): >2.5 mm* from base of polyp (surrounding mucosa)
2.
Flat lesions (Fig. 6.2)
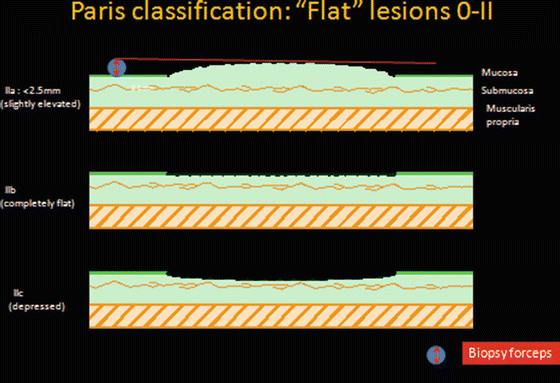

Fig. 6.2
Paris Japanese classification for flat lesions
(a)
IIa: Slightly elevated (<2.5 mm*)
(b)
IIb: True flat lesion
(c)
IIc: Mildly depressed lesion
*The 2.5 mm limit is used to differentiate sessile (Is) from flat (0-IIa) lesions and approximates the diameter of a closed biopsy forceps.
Flat colorectal lesions account for up to half of all colorectal polyps, while depressed lesions occur less frequently and account for about 1–3 % of all polyps (Table 6.2). The prevalence of high-grade dysplasia (HGD) or invasive cancer, however, increases as lesions become depressed (Paris IIc). Up to 59 % of all Paris type IIc lesions harbor HGD or submucosal invasion (SMI) (Table 6.3).
Table 6.2
Percentage of flat-depressed colorectal lesions
Author | No. of adenomas | % of flat lesions |
|---|---|---|
Jaramillo [10] | 261 | 42 |
Rembacken [11] | 321 | 36 |
Saitoh [12] | 136 | 40 |
Rex [1] | 785 | 56 |
Okuno [13] | 66,670 | 1.9 |
Togashi [14] | 5,408 | 2.8 |
Soetikno [15] | 1,535 | 1.2 |
Tsuda [15] | 973 | 1.4 |
Table 6.3
Prevalence of HGD and SMI in colorectal polyps according to Paris Japanese classification
Author | HGD | SMI | ||||
|---|---|---|---|---|---|---|
Ip | IIa/b | IIc | Ip | IIa | IIc | |
Rembacken [11] | 7.4 % | 12.8 % | 25 % | 0.9 % | 1.7 % | 50 % |
Soetikno [15] | 0.5 % | 3.5 % | 225 | 0.5 % | 1.0 % | 11 % |
Tsuda [16] | 7.3 % | 12.8 % | 35.7 % | – | – | – |
Hurlstone [17] | 12 % | 15.4 % | 59 % | – | – | – |
Large colorectal lesions (measuring >20 mm in size) are relatively infrequent and may account for up to 4 % of all polyps (Table 6.4). The size of the lesion does not appear to matter when lesions are assessed for SMI, though. Moss and colleagues prospectively assessed colorectal LSTs in a large cohort of Australian patients; SMI was detected in 33/514 (6.4 %) polyps. The mean size of these polyps was 35.3 mm, in comparison to 35.6 mm when no SMI was detected (p = 0.87) [18].
Table 6.4
Comparison of Paris Japanese classification and size
Total | ≤5 mm | 6–10 mm | 11–19 mm | ≥20 mm | |
Polypoid (Is, Ip) | 14,814 | 47.6 % | 37.7 % | 12.6 % | 2.1 % |
Flat (IIa, IIb) | 10,363 | 73.1 % | 13.9 % | 9.0 % | 4.0 % |
Flat-depressed (IIc) | 585 | 45.0 % | 29.4 % | 21.7 % | 3.9 % |
Total | 24,862 | 14,892 | 7,190 | 2,919 | 761 |








