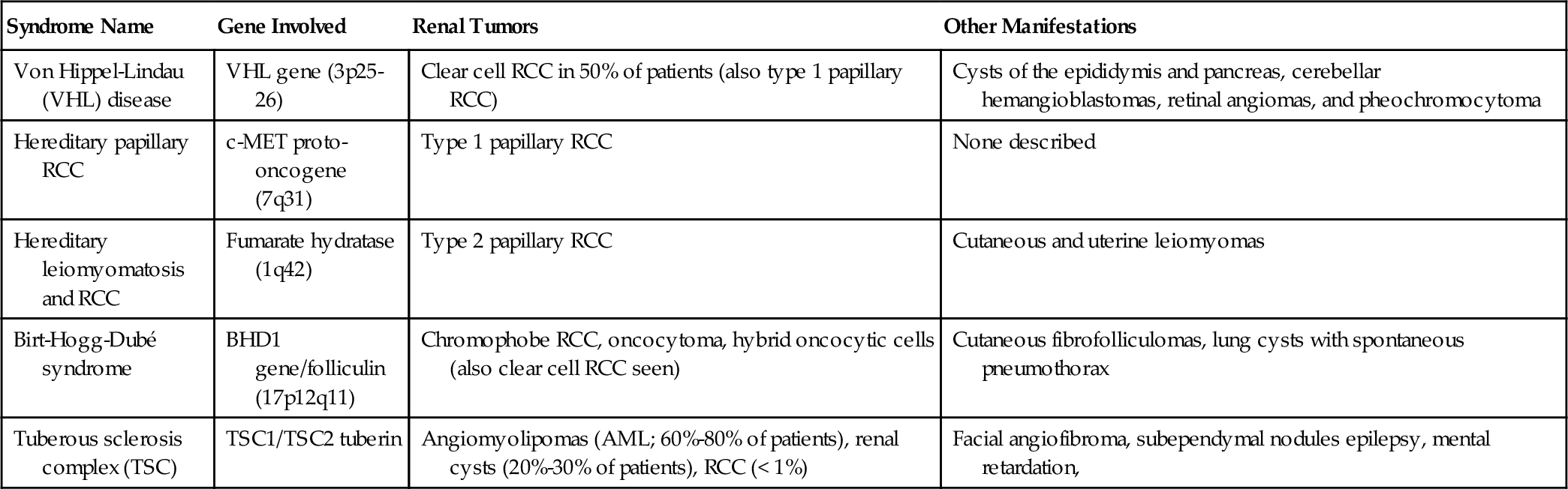
Chapter 17 Eugene J. Pietzak, III, MD; Thomas J. Guzzo, MD, MPH; Alan J. Wein, MD, FACS, PhD (hon); Matthew J. Resnick, MD The paradigm in diagnosis, evaluation, and management of renal cell carcinoma (RCC) has evolved significantly over the last several decades. Whereas 30 years ago the diagnosis of RCC was predicated on clinical signs and symptoms, the majority of renal tumors are now diagnosed incidentally on imaging performed for other reasons. The identification of iatrogenic renal insufficiency associated with radical nephrectomy has rendered nephron-sparing techniques the preferred treatment for small renal masses. Active surveillance of small renal masses has also emerged as an option for select patients. Although RCC remains the prototype for tumors resistant to radiation and chemotherapy, an expanding number of molecular targeted therapies are now available. There will be approximately 63,150 new cases of RCC in 2013 and 13,680 deaths attributable to the disease. The incidence of RCC has increased over the last several decades with a stage migration towards lower-stage, smaller tumors. Despite this downward stage migration, there has yet to be a demonstrable decrease in kidney cancer-related mortality rates. RCC represents 5% of all male cancers and 3% of all female cancers, with a male-to-female ratio of 3:2 and a peak incidence in the sixth to seventh decade of life. Most RCC cases are sporadic, but a number of hereditary conditions are associated with various renal tumors (Table 17-1). In general, RCC in hereditary syndromes have an autosomal-dominant inheritance pattern with variable penetrance. RCCs associated with hereditary syndromes generally present at an earlier age and are more likely to be bilateral and multifocal. Although these hereditary conditions are uncommon (2% to 3% of all patients with RCC), they have been essential in improving RCC tumor biology. Tobacco use is the strongest known risk factor for RCC, with users having an approximately twofold increased risk. Hypertension and obesity have also been associated with RCC in large epidemiologic studies. Furthermore, increased incidence of RCC has been reported in patients with acquired renal cystic disease secondary to end-stage renal disease (ESRD)/dialysis. Given this observed increased risk, many practitioners advocate performing periodic imaging in dialysis patients. b. Clear cell (conventional) RCC: This is the most common histology accounting for 70% to 80% of all RCC. It is associated with an allelic loss of the VHL gene. Grossly, tumors appear yellow or gray-tan in color with variable areas of hemorrhage, necrosis, or cystic changes. Microscopically, they are composed predominantly of cells with clear eosinophilic or granular cytoplasm. In general, clear cell RCC has a worse prognosis than papillary and chromophobe RCC after adjustment for grade and stage. c. Papillary RCC: This comprises 10% to 15% of newly diagnosed RCC, but accounts for a higher percentage in patients with end-stage and acquired renal cystic disease. Papillary RCC is more likely to be multifocal or bilateral than other histologies. Two distinct subtypes are recognized: Type 1 and Type 2 papillary RCC. They are associated with two different familial RCC syndromes (see Table 17-1). Papillary type 1 tumors are associated with activation of the MET proto-oncogene (chromosome 17), whereas papillary type 2 tumors are associated with mutations in the fumarate hydratase gene. Type 2 papillary RCC tends to be the more aggressive variant. d. Chromophobe RCC: This represents 3% to 5% of RCC and is associated with multiple chromosome losses. Histologically, a defining feature is its perinuclear “halo.” Chromophobe RCC histology may be confused with oncocytoma, a benign renal lesion. Chromophobe RCC tends to have a better prognosis than clear cell and papillary subtypes. e. Collecting duct carcinoma: This is a rare but very aggressive RCC subtype, representing less than 1%. It tends to occur in younger patients, and up to half have metastasis at presentation. f. Renal medullary carcinoma: This is a highly aggressive tumor seen in young black Americans with sickle cell trait. It may represent a variant of collecting duct carcinoma and carries a very poor prognosis. 2. Oncocytoma 3. Angiomyolipoma (AML) 4. Sarcoma 5. Lymphoma 6. Metastasis The Fuhrman grading system is commonly used to classify nuclear features of RCC. It ranges from Grade I (well differentiated) to IV (poorly differentiated) and is contingent on nuclear size, nuclear outline, and presence of nucleoli. The most common staging system for RCC remains the American Joint Committee on Cancer (AJCC) TNM system (Table 17-2). Major changes occurred in the tenth edition, including subdivision of T2 tumors and reclassification of tumors with lymphatic involvement, tumor thrombus, and adrenal metastasis. Pathologic stage remains the single most important prognostic factor. Other important considerations are tumor size, grade, subtype, presenting symptoms, and patient factors. Whereas 5-year survival for patients with T1a disease is between 90% and 100%, survival drops to 50% to 70% in patients with T3a disease, and to 20% or less in patients with nodal or distant metastatic disease. Greater than 50% of all RCCs are found as asymptomatic incidental masses on imaging studies obtained for another purpose. The classic triad of hematuria, flank pain, and a palpable mass was historically seen in approximately 10% of patients but is now a rare presentation. Systemic symptoms of unintentional weight loss, fever, anemia, and night sweats suggest the presence of metastasis. Tumor thrombus can obstruct drainage of the testicular vein, producing a right-sided or noncollapsible varicocele. Paraneoplastic syndromes may occur in advanced disease, including hypercalcemia from parathyroid-like hormone production, hypertension, polycythemia, and Stauffer syndrome, or nonmetastatic hepatic dysfunction. The most common renal mass is a benign cyst, identified in approximately 50% of patients over 50 years of age. A mass not meeting ultrasound criteria for a simple cyst should be evaluated with a dedicated renal CT scan or MRI with thin cuts. Renal cysts are classified by the Bosniak system (Table 17-3). Table 17-3 Bosniak Classification of Cystic Masses b. Renal ultrasound: Ultrasound is an inexpensive and noninvasive modality used to distinguish cystic from solid renal masses. A simple cyst will be smooth with a definite border of imperceptible thickness, be absent of internal echogenicity, and should display acoustic enhancements beyond the posterior wall. c. MRI: This is an alternative to CT for evaluation of renal masses and is particularly useful for those with iodinated contrast allergies. Enhancement seen after gadolinium contrast on T1-weighted images is characteristic of RCC. MRI is also effective in demonstrating the presence and extent of renal vein or vena caval tumor thrombi. Whereas contrast-enhanced MRI was historically performed in patients with renal insufficiency, it is now avoided because of the risk of nephrogenic systemic fibrosis with gadolinium administration in patients with chronic kidney disease (CKD). d. Angiography: Classic angiography has been largely replaced by magnetic resonance angiography or 3D CT angiography in the evaluation of the renal vessels. However, renal angioembolization may be useful before radical nephrectomy for very large central tumors or in the presence of bulky hilar adenopathy. e. Radionuclide imaging: This may be useful in determining the presence of a pseudotumor. Renal pseudotumors can results from multiple causes, but the most common is hypertrophied column of Bertin. Pseudotumors appear with a uniform distribution of radioisotope uptake on radionuclide imaging, whereas benign cysts and true renal tumors will appear as photon-deficient areas of uptake. f. Percutaneous biopsy: Historically, biopsy was performed only if a renal mass was suspected to be lymphoma, metastasis, or abscess. In recent years, percutaneous biopsy has been revisited. Nearly one third of small renal masses are benign, and the majority of the remaining two thirds are frequently low-grade tumors. Most contemporary series show greater than 90% accuracy with biopsy, with complication rates less than 2%. Biopsy provides valuable information on grade and histologic subtype, both of which can be used for treatment planning. 2. Clinical staging 1. Small renal masses and localized disease (T1-T2) b. Tumor ablative techniques c. Active surveillance 2. Locally advanced RCC b. Adjuvant systemic therapy: No studies of adjuvant systemic therapy after nephrectomy for high-risk patients have demonstrated a survival advantage. These trials have been limited by patient accrual. 3. Metastatic RCC b. Cytoreductive nephrectomy: The role for radical nephrectomy in patients with metastatic RCC in the current era of targeted systemic therapy remains largely unknown. Historically, cytoreductive nephrectomy was performed in individuals with an excellent performance status undergoing immunotherapy. Randomized trials have demonstrated a benefit of nephrectomy and immunotherapy compared to immunotherapy alone; however, no such trials have been reported in patients receiving targeted therapy. c. Metastasectomy: Approximately 1% to 4% of patients with metastatic disease will have a solitary metastasis or oligometastasis, for which surgical resection provides a 5-year survival between 30% and 50%. d. Systemic therapy: The chemo-refractory nature of RCC likely arises from the expression of multidrug resistance efflux pump proteins. RCC is, however, an immunogenic tumor, and the use of immunotherapy was the cornerstone of systemic therapy for many years. Interferon alpha provides a 15% partial response rate, but complete response is very rare; therefore it is no longer commonly used. High-dose interleukin-2 remains the only treatment associated with durable, complete response with long-term survival in select patients. A response rate in the 10% to 20% range has been demonstrated, but treatment is associated with major toxicities, including adult respiratory distress syndrome (ARDS) as a result of the capillary leak syndrome. Some centers still offer high-dose interleukin-2 to young patients with good performance status who have metastatic clear cell RCC. Inpatient admission, frequently to ICU-level settings, is required during administration to monitor for toxicity and provide supportive care. Practice patterns for RCC follow-up vary dramatically between centers. Because cross-sectional imaging is usually required, cost and radiation exposure can vary widely between surveillance protocols. The NCCN recommends a history, physical, and comprehensive metabolic panel every 6 months for 2 years, then annually for 5 years. According to the NCCN, imaging of the chest, abdominal, and pelvis should be obtained 2 to 6 months from treatment and then only as indicated. The authors advocate for an individualized approach based on patient and tumor characteristics. The urinary tract is lined by urothelial cell epithelium from the most proximal calyces to the proximal urethra. In this section, attention is given to the tumors of the renal collecting system and the ureter. It is essential that urothelial tumors of the renal pelvis and ureter be understood in the broad context of urothelial cell carcinoma (UCC), which is discussed in greater detail in the section on bladder cancer. Although UCC of the upper tract shares similarities to urothelial carcinoma of the bladder (UCB), several distinct differences merit consideration. Upper tract UCC (UTUCC) accounts for 5% to 7% of all renal tumors and 5% to 10% of all urothelial cancers. The exact incidence of UTUCC is largely unknown because renal pelvis tumors are generally included in data on all renal tumors. It is estimated that there will be 2,710 new cases of ureteral UCC in 2013 with 900 deaths attributable to the disease. Although the incidence of UTUCC is thought to be increasing over the last several decades, it remains a rare tumor type. Patients with a history of UCB have a 2% to 4% chance of developing upper tract tumors (synchronous or metachronous); however, this increases up to 25% in patients with carcinoma in situ (CIS) of the bladder or in patients with high-grade tumors adjacent the ureteral orifices. Following radical cystectomy, an approximately 7% chance of developing UTUCC exists, with the preponderance of this risk being in the first 3 to 4 years after cystectomy. Patients with high-grade nonmuscle-invasive bladder cancer should undergo routine upper tract surveillance, given the increased risk of developing upper tract disease. Nonetheless, the optimal intensity of upper tract surveillance remains largely unknown. It is the authors’ practice to perform upper tract surveillance every 1 to 2 years in high-risk patients. Conversely, patients with a history of an incident upper tract tumor have a 15% to 50% chance of eventually developing UCB. For this reason, routine surveillance cystoscopy is necessary in all patients with UTUCC. Metachronous bilateral UTUCC occurs in 2% to 4% of patients with UTUCC. As in UCB, chemical carcinogenesis is the most important factor in the development of UTUCC. Because of the relatively short transit time of urine through the upper tracts, lesions of the upper tract occur much less often than bladder lesions. Similarly, distal ureteral tumors are more common than proximal. Ureteral UCC occurs in the distal, mid, or proximal ureter in 70%, 20%, and 5% of cases, respectively. Similar to UCB, the most common risk factor is smoking. Tobacco use is associated with at least a twofold increase in the relative risk for the disease, but this risk may be up to sevenfold higher in long-term, heavy smokers. An uncommon but notable risk factor for the development of UTUCC is Balkan nephropathy, an inflammatory lesion of the renal interstitium endemic to the Balkan region. Balkan nephropathy is characterized by multiple, often bilateral, low-grade upper tract urothelial tumors that may be associated with aristolochic acid intake. Abuse of phenacetin, an analgesic medication withdrawn by the FDA, is considered a historical risk factor for the disease. A few hereditary conditions are associated with UTUCC, including Lynch syndrome II, which is characterized by the early development of nonpolyposis colonic tumors and additional extracolonic neoplasms. Depth of invasion is the single most important prognostic factor in UTUCC 5-year survival ranges from 100% for noninvasive tumors (Ta) to 40.5% for tumors invading beyond the muscularis (T3). Because of the relative paucity of muscle wall thickness compared to the bladder, UTUCC is considered more likely to be of advanced stage at presentation. Interestingly, the renal parenchyma is thought to prevent spread of T3 tumors in the renal pelvis compared to UTUCC in the ureter. Sixty percent to 98% of patients present with either microscopic or gross hematuria. Flank pain can occur in up to 30% of patients, which may be due to obstruction secondary to tumor, blood clot, or a combination of both. Only 15% of UTUCC is identified incidentally; however, these tumors are likely to manifest clinically because UTUCC is rarely discovered at autopsy. UTUCC and UCB share common pathologic subtypes. The TNM staging systems for UTUCC is analogous to that of UCB (Table 17-4). However, clinical staging in UTUCC is extremely difficult given the technical challenges associated with endoscopic sampling. Table 17-4 AJCC 7th Edition Staging System for Upper Tract Urothelial Carcinoma Primary Tumor (T) TX – Primary tumor cannot be assessed T1 – Tumor invades subepithelial connective tissue T2 – Tumor invades the muscularis T3 – Renal pelvis tumor invades beyond muscularis into peripelvic fat or the renal parenchyma Regional Lymph Nodes (N)* NX – Regional lymph nodes cannot be assessed N0 – No regional lymph node metastasis Distant Metastasis (M) M0 – No distant metastasis M1 – Distant metastasis (From Edge SB, Byrd DR. Compton CC, et al, editors: AJCC cancer staging manual, ed 7, New York, 2010, Springer.) * Note: Laterality does not affect the N classification.
Adult Genitourinary Cancer
Renal, Testicular, and Penile
Neoplasms of the kidney
General considerations
Incidence and epidemiology
Etiology
Pathology: renal tumors
Accounting for approximately 5% of all renal tumors, oncocytomas are well-circumscribed parenchymal masses composed of densely acidophilic cells that show mitochondrial hyperplasia on electron microscopy. They may be bilateral in up to 5% of cases. In contrast to RCC, the principal genetic alteration appears to involve changes in mitochondrial DNA and translocation of chromosome 14. Grossly, they are mahogany brown in color, encapsulated, and may contain a dense central scar extending in a stellate pattern, which may be identified in cross-sectional imaging. Oncocytomas are benign and can be managed conservatively; however, their preoperative clinical diagnosis is often unreliable, and a definitive diagnosis may be made on frozen section or percutaneous biopsy due to histologic similarities to chromophobe RCC.
These benign lesions composed of fat, muscle, and blood vessels can generally be identified by the presence of macroscopic fat on CT scan. Although lesions are generally identified as incidental radiographic findings, patients may present with acute flank pain or hemorrhagic shock due to spontaneous renal or retroperitoneal hemorrhage. Treatment is based on tumor size and patient symptoms. Asymptomatic tumors smaller than 4 cm may be managed expectantly. However, symptomatic tumors or those greater than 4 cm should undergo selective embolization, percutaneous ablation, or nephron-sparing surgery. Some large central tumors not amenable to nephron-sparing strategies may require radical nephrectomy. Recently, mammalian target of rapamycin (mTOR) inhibitors have been shown to decrease the size of AML and may be a promising therapy for patients with multiple lesions.
Sarcomas constitute 2% to 3% of malignant renal parenchymal tumors. They are more common in women. Differentiation from RCC is difficult. The predominant subcategory is leiomyosarcoma. The preferred treatment remains radical nephrectomy with wide negative margins.
B-cell non-Hodgkin lymphoma will occasionally present as an infiltrative renal mass. It is uncommon for leukemia to present as a primary renal lesion.
The most common metastases to the kidneys are primary carcinomas of the lung, breast, and uterus, and melanoma. Metastatic lesions appear poorly vascularized and display irregular borders on imaging studies. A percutaneous biopsy of the renal mass may be warranted in patients with signs of progression from their other malignancy or in masses that do not demonstrate contrast enhancement on imaging studies.
Grading and staging
Clinical presentation
Imaging evaluation
Category
Description
Risk of Malignancy
Bosniak I
Simple cyst; measuring water density; does not enhance; contains no calcifications
< 2%
Bosniak II
Minimally complex cyst; thin wall (< 1 mm); no enhancement, although may contain several hairline septa; hyperdense cyst
< 5%
Bosniak IIF
Indeterminate; complex cyst with thicker septa
~ 25%
Bosniak III
Suspicious indeterminate; thicker, regular, nodular walls with thicker, regular calcifications and septations
~ 50%
Bosniak IV
Malignant; nodular or solid component
> 90%
The initial workup and clinical staging, according to the National Comprehensive Cancer Network (NCCN) guidelines, consists of a history, physical exam, complete blood count, comprehensive metabolic panel, urinalysis, chest imaging, and a contrast-enhanced cross-sectional imaging of the abdomen and pelvis. A bone scan may be obtained in symptomatic patients or those with elevated alkaline phosphatase. If neurologic symptoms or extensive metastatic disease is present, a brain MRI should be considered. For renal masses that are suspicious for urothelial origin, dedicated urothelial imaging (including urogram or pyelograms), urinary cytology, and ureteroscopy should be considered.
Treatment
Percutaneous or laparoscopic ablation using cryosurgery or radiofrequency offers reduced morbidity and more rapid recovery when compared to surgical excision. These benefits, however, are balanced with higher rates of local recurrence. Between-series comparisons are difficult because reliable histologic and pathologic staging is not performed with ablative techniques. Long-term efficacy has yet to be established, and, as such, ablation is typically reserved for patients of advanced age and significant comorbidity. Tumor size is also an important predictor of success; therefore ablative therapy is generally reserved for renal masses less than 4 cm.
With the increasing number of incidentally identified renal masses, particularly in comorbid elderly patients, active surveillance for small renal masses has emerged as a reasonable management strategy. The median growth rate for most small renal masses is less than 0.3 cm/year, and, for biopsy-proven RCC, it is only approximately 0.4 cm/year. Tumors less than 3 cm may be monitored with serial imaging every 6 to 12 months. Increased growth kinetics and surpassing a size threshold are typical indications for intervention. Early series demonstrate a low risk of metastatic dissemination in small renal masses under active surveillance.
In recent years, efforts have focused on leveraging knowledge of the molecular mechanisms by which RCC develops. This has led to the usage of molecular targeted therapies that inhibit angiogenesis and cell cycle regulation. Tyrosine kinase inhibitors (TKIs) that target VEGF-mediated pathways are most commonly used. Sunitinib was originally used in immunotherapy refractory patients but now is most commonly used instead of immunotherapy as a first-line agent. Sorafenib, another tyrosine kinase inhibitor that is thought to be more promiscuous with the receptors it targets, is frequently given as a second-line agent after progression on a first TKI. Additional kinase inhibitors are now available; however, the optimal sequencing of these agents is unknown. Everolimus is an mTOR inhibitor reserved as a second-line therapy for patients refractory to tyrosine kinase inhibitors. Temsirolimus, another mTOR inhibitor, is approved for poor-risk metastatic RCC. Using RECIST criteria, multiple studies have revealed fairly consistent response rates to various TKIs, on the order of 30% to 40%. Despite the fact that these agents are largely well-tolerated and provide satisfactory disease control, there have been few long-term responses to TKI therapy. Further investigation of combination therapy is ongoing.
Follow-up after treatment
Urothelial tumors of the renal pelvis and ureter
General considerations
Incidence
Etiology and natural history
Clinical presentation
Pathology and staging
T0 – No evidence of primary tumor
Ta – Papillary noninvasive carcinoma
Tis – Carcinoma in situ
T3 – Ureter tumor invades beyond muscularis into periureteric fat
T4 – Tumor invades adjacent organs or through the kidney into perinephric fat
N1 – Metastasis in a single lymph node 2 cm or less in greatest dimension
N2 – Metastasis in single lymph nodes > 2 cm but not > 5 cm in greatest dimension; or multiple lymph nodes, none > 5 cm in greatest dimension
N3 – Metastasis in a lymph node > 5 cm in greatest dimension
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree