Fig. 14.1
Adenomatosis in a 37-year-old female. Axial non-fat-saturated T1-weighted image (a) shows multiple hypointense masses (arrows). The presence of bright signal in one of the masses (arrowhead) suggests the presence of hemorrhage. Opposed phase image (b) shows loss of signal in most of the lesions (arrows), suggesting the presence of lipid. These lesions are hypervascular in the arterial phase (arrows), post gadolinium injection (c), and washout in the portal venous phase (d). These MR findings are consistent with adenomatosis
In the past decade, improvements in the pathological characterization of HA have led to a more mechanistic understanding of the etiology of HA. There are now recognized distinct HA subtypes described by the World Health Organization [9, 11]. Correspondingly, each subtype demonstrates variable imaging, clinical behavior, and natural history [11].
While different classification schemes have been developed, the most accepted HA subtype designations include: (1) β-catenin-mutated HAs (HA with cellular atypia), (2) hepatocyte nuclear factor-1-α-mutated HAs (steatotic HAs), (3) inflammatory HAs (telangiectatic HA), and (4) unclassified. All of these subtypes may present as solitary lesions to multiple lesions.
β-catenin-mutated HAs represent 10–15 % of all HAs [9, 12]. They are usually solitary and are disproportionately more common in men [8]. With mutation of the β-catenin gene, there is inappropriate upregulation of many of its targets, including glutamine synthetase and the G-protein-coupled receptor 49 [13]. This subsequent aberrant upregulation contributes to the development of cellular atypia and an increased risk of malignant transformation to HCC [11]. Furthermore, the histological distinction between these lesions and HCC can be challenging, and some groups have recently designated some of these lesions as well-differentiated HCC [12].
Hepatocyte nuclear factor-1-α (HNF1α)-mutated HAs are found in 30–50 % of HA patients, with a predisposition in younger women. These often demonstrate evidence of steatosis, and there is an absence of cellular atypia [14]. HNF1α HAs are caused by biallelic mutations in the transcription factor HNF1α [15] resulting in inappropriate hepatocyte differentiation. Adenomatosis in HNF1α-mutated HAs often contain a germline mutation in the HNF1α gene [16]. Emerging evidence also suggests that there is an association with HNF1α adenomatosis and a personal or family history of MODY3 diabetes [5].
The inflammatory HA is the most common, comprising up to 50 % of all HAs, and is also the most directly associated with OCP [13]. Histologically, inflammatory HA has sinusoidal dilation, thickened arteries with absent veins and biliary ducts, and inflammatory infiltrates [17]. Whether an inflammatory event leads to these HAs or whether the HA triggers an inflammatory cascade is unclear. However, in patients with inflammatory HA, activating mutations have been identified in the interleukin-6 receptor gene and Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway [18]. There is also a characteristic high expression of C-reactive protein and the inflammatory marker serum amyloid-associated (SAA) protein [18]. Furthermore, inflammatory HA is unique in that obesity and alcohol abuse–associated steatohepatitis are associated with the development of this type of HA [19].
The remaining HAs are categorized into the unclassified HA subtype. These unclassified HAs represent up to 10 % of all HAs, and they do not demonstrate any particular histological, morphological, or genetic features [9, 20].
14.1.3 Clinical Presentation
Most HAs are asymptomatic and are discovered incidentally. A subset of patients will present with pain. Another presenting symptom associated with HA includes bleeding – manifesting as acute pain or frank hemorrhage with symptomatic anemia. In general, tumors larger than 5 cm have an increased risk of bleeding and rupture, with up to 11–49 % of all large HAs having bleeding complications [21–23]. Not unexpectedly, the risk of bleeding and malignancy varies based on the subtype. Inflammatory HAs are the most prone to bleeding, with 30 % associated with a bleeding complication. The risk for bleeding is likely a manifestation of sinusoidal dilation and abnormal arterial vasculature [11, 21]. In contrast, HNF1α HAs have a minimal risk of bleeding complications, while the incidence of β-catenin-mutated HA bleeding episodes is unknown [20].
Another possible complication of HA is malignant transformation to HCC. Overall, the risk of HCC is 4–10 %, with risk factors being male sex, anabolic steroid use, and size greater than 5 cm [23–25]. Similar to the risk of bleeding, the risk of malignant transformation varies based on the HA subtype. The subtype most associated with HCC is the β-catenin-mutated HA [10]. This is not surprising as the β-catenin pathway is mutated in approximately 35 % of HCC [26]. While the β-catenin-mutated group has the highest risk of HCC, 10 % of inflammatory HAs also have an increased risk of malignancy, while the risk with HNF1α HA is minimal [11, 20, 21].
14.1.4 Evolution and Therapy
Most patients present with HA detected on imaging performed for other reasons, such as discomfort, stable anemia, or as an incidental finding. The best imaging modality to establish the diagnosis of HA is probably MRI. On MRI most HAs typically have a characteristic isointense or hyperintense appearance on T1- and T2-weighted images. Uniform enhancement on the arterial phase and washout and isointensity with the background liver on the portal phase are also characteristic. Based on the subtype, enhancement patterns vary (Table 14.1). If after MRI the diagnosis is still in question, biopsy may be warranted.
Table 14.1
MRI characteristics based on hepatic adenoma subtypes
Subtype | T1-weighted gradient-echo MR images | T2-weighted MR images | Gadolinium-enhanced T1-weighted MR images |
|---|---|---|---|
Inflammatory HA | Isointense or hyperintense, without signal drop-off with use of chemical shift sequence | Diffusely hyperintense | Intense enhancement during arterial phase that persists in the portal venous and delayed phases |
HNF1α-mutated HA | Isointense or hyperintense, with diffuse signal drop-off with the use of chemical shift sequence | Isointense to slight hyperintense | Moderate enhancement in the arterial phase, with no persistent enhancement in the portal venous and delayed phases |
β-catenin-mutated HA | No specific MR imaging patterns – strong enhancement during arterial phase, with portal venous washout | No specific MR imaging patterns – strong enhancement during arterial phase, with portal venous washout | No specific MR imaging patterns – strong enhancement during arterial phase, with portal venous washout |
The management of HA is based on the presentation. Resection is offered to patients with symptoms or with risk factors for bleeding or malignant transformation, such as patients with large lesions. Risk factors associated with bleeding and HCC transformation include a history of bleeding, size approaching 5 cm, or concern on imaging or biopsy for HCC [23, 24]. Other relative indications for resection are those patients at risk for malignant transformation – those with glycogen storage diseases, male sex, history of androgen or steroid intake, or concerns by imaging or biopsy of a β-catenin-mutated HA [4, 6, 11]. In women with HA less than 5 cm and taking OCPs, some groups recommend cessation of OCPs and re-imaging at 6–12 months, as regression is possible [27]. However, improvement in symptoms after taking OCPs is unusual and does not eliminate the risk of malignant transformation or hemorrhage, and careful surveillance is warranted. In the patient with an acutely bleeding HA, interventional radiological (IR) guidance with hepatic artery embolization of the feeding hepatic artery is the treatment of choice, reserving open hepatectomy for a later time when the bleeding has been controlled by IR therapies [28].
14.2 Biliary Cystadenoma
14.2.1 Epidemiology
Biliary cystadenomas (BCAs) are rare epithelial tumors which account for less than 5 % of all cystic liver lesions [29]. BCAs occur predominantly in females and generally present during the fourth or fifth decade of life [30, 31]. Besides female gender, there are no other clearly defined risk factors although the use of OCPs have been postulated in some reports to be associated with BCA [32, 33]. BCA tumors are classified according to the presence of ovarian-like stroma. Unlike BCAs with ovarian stroma, BCAs without ovarian stroma occur equally in both genders. Non-ovarian BCA tumors may, however, occur at a more advanced patient age.
14.2.2 Etiology
Nearly 90 % of all BCAs are derived from the intrahepatic biliary tree, while 10 % originate from extrahepatic biliary structures [32, 36]. The etiology of BCA remains unclear, and these tumors have been described as both congenital and acquired lesions [37]. BCAs are believed to derive from ectopic rests of embryonic bile ducts, ovarian cells, or intrahepatic peribiliary glands [32, 38]. BCAs can be premalignant and develop into biliary cystadenocarcinoma (BCAC), which are more likely to occur in older, male patients [31, 32]. In fact, Devaney et al. hypothesized that BCAC originates from either a benign BCA with ovarian stroma or de novo, occurring exclusively in men [32].
Although Hueter first reported BCAs in 1887, Edmonson originally characterized this tumor in 1958 [39]. Wheeler and Edmondson later reclassified BCAs in 1985 according to the following distinct criteria: multilocular cysts, columnar epithelium, and the presence of ovarian stroma [38]. Devaney and colleagues [32] revised this definition in 1994 since 14 % of the cystadenomas in their series of 70 patients did not contain ovarian stroma. Moreover the authors noted that biliary cystic tumors could also be unilocular and therefore suggested that multilocularity not be a qualifying feature of biliary cyst tumor classification [32].
14.2.3 Clinical Presentation
The clinical presentation of BCA is varied. Common patient complaints include nonspecific symptoms such as abdominal pain, abdominal distention, early satiety, and weight loss – although many patients are asymptomatic at the time of diagnosis [30, 35]. Obstructive jaundice and cholangitis are rarely seen and are generally associated with extrahepatic BCA.
More liberal use of cross-sectional imaging as well as the improved sensitivity of imaging techniques has led to increased incidental findings of BCAs. Ultrasound is the most common imaging modality used to characterize these lesions [30]. BCAs typically appear anechoic and internal septations are generally seen [36]. Computed tomography (CT) with intravenous contrast results in nodular enhancement of cystic lesions which are isodense to water. T1- and T2-weighted magnetic resonance imaging often demonstrates a low-intensity T1 signal and high intensity T2 signal [36] (Fig. 14.2).
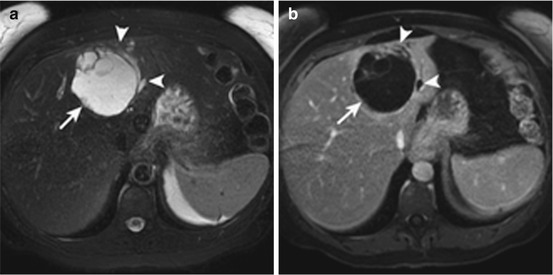

Fig. 14.2
Biliary cystadenoma in a 53-year-old female. Axial T2-weighted image (a) and post contrast T1-weighted image in the portal venous phase (b) show a large complex mass with thick enhancing septations (arrow). There is associated left ductal dilatation (arrowheads). These MR findings are consistent with biliary cystadenoma
Although definitive differentiation between BCA and BCAC is not possible with current imaging techniques, certain features are associated with a higher likelihood of malignancy such as the presence of intracystic debris, bile duct dilatation, and mural nodules [34, 36]. CT and MRI demonstrate the anatomic relationships of surrounding structures including hepatic vasculature as well as any biliary dilatation. Cross-sectional imaging is critical for preoperative planning. Magnetic resonance cholangiopancreatography (MRCP) can demonstrate biliary communication and can aid in differentiating BCA from simple cysts through visualization of mural nodules and septations. The role of endoscopic retrograde cholangiopancreatography in BCA is limited and typically only used when tissue diagnosis is needed.
The differential diagnosis is broad and includes simple cyst, hemangioma, adenoma, hepatic abscess, hemorrhagic cyst, and hydatid cyst. Appropriate therapeutic interventions for each of these entities are varied; therefore, precise preoperative identification of BCA leads to a lower risk of recurrence and malignant transformation. Cysts without internal septations or papillary projections with a well-defined tissue fluid interface are more likely simple hepatic cysts and can be managed conservatively [40, 41]. Daughter cysts are pathognomonic of echinococcal cyst, and fine-needle aspiration in conjunction with serologic analysis is diagnostic [41, 42]. Similarly for liver abscesses, an irregular, contrast-enhanced, thicker cyst wall is common and cyst fluid culture is diagnostic [41].
Measurement of serum tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) has not proven diagnostic in cases of biliary cystic tumors and is therefore not utilized for clinical differentiation. Core needle biopsy, fine-needle aspiration, and cyst fluid analysis of BCAs remain controversial. CA 19-9 and CEA cyst fluid levels have low sensitivities and specificities and cannot be used to rule out malignancy. Additionally, tissue sampling and cytology are rarely diagnostic, and dissemination of malignant cells with these procedures has been reported [40, 43]. Therefore, the associated low diagnostic yield and potential oncologic ramifications make FNA and core needle biopsy of suspected BCAs relatively contraindicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







