Chapter 48 Acute Relapsing Pancreatitis
Introduction
Acute pancreatitis is caused by acute or chronic alcohol intake or cholelithiasis in 80% of cases.1,2 In the absence of alcohol or gallstones, numerous established and putative etiologies must be considered, any one of which can cause recurrent attacks of acute pancreatitis.3 In instances in which the underlying etiology eludes detection and leads to a second attack, the term acute relapsing pancreatitis (ARP) is applied. Table 48.1 lists the etiologies of ARP categorized by entities typically managed medically versus entities that respond to endoscopic therapy to prevent recurrences. This chapter focuses on the etiologies of ARP responding to endoscopic therapy. We also provide a brief update on the newly discovered and expanding body of knowledge of genetic causes of ARP, autoimmune ARP, and celiac-associated ARP. Endoscopic management for the genetic conditions is usually reserved for complications that develop from chronic pancreatitis (CP) and is discussed in Chapter 49. For a summary of all etiologies of ARP, readers are directed to the comprehensive review of Somogyi and coworkers.4
Table 48.1 Putative Etiology of Acute Relapsing Pancreatitis
| Medical Management | Endoscopic or Surgical Management |
|---|---|
| Alcohol | Annular pancreas |
| Autoimmune | Biliary stones or microlithiasis |
| Celiac disease | Choledochocyst and choledochocele |
| Drug-induced | Pancreas divisum |
| Genetic | Pancreatic and ampullary neoplasms |
| Hereditary pancreatitis CFTR mutations SPINK mutations Tropical pancreatitis | Periampullary diverticulum |
| Sphincter of Oddi dysfunction | |
| Hypercalcemia | |
| Hyperlipidemia | |
| Infectious | |
| Vascular |
An initial complete history and physical examination, routine blood work including liver function tests and corrected or ionized calcium and triglyceride levels, and transabdominal ultrasound or computed tomography (CT) scan reveal an etiology in 70% to 90% of cases of pancreatitis.2,5–8 In younger patients (<40 years old), transabdominal ultrasound may suffice, but in older patients, a CT scan of the abdomen is advised because a pancreatic or ampullary neoplasm may manifest with ARP.9,10 Without an adequate initial work-up and directed therapy, more than half of patients with an initial attack of acute pancreatitis experience recurrent attacks or develop CP.5,11 In patients with gallstone pancreatitis, treatment of the index attack and prevention of further attacks may involve endoscopic sphincterotomy (ES) with bile duct stone extraction and laparoscopic cholecystectomy to remove the stone reservoir.6,7 Patients who remain untreated have a 33% to 66% chance of a recurrent attack.8,11–13
Work-up of Acute Relapsing Pancreatitis
After an initial unrevealing evaluation for acute pancreatitis, ERCP reveals an etiology in approximately 70% of patients.5,14 Although some experts advocate performing ERCP in all patients after a single attack of pancreatitis, most agree that ERCP is warranted only after a severe attack in which the etiology is not obvious or after recurrent attacks.15,16 The utility of this test lies in its unique ability to diagnose and treat biliary microlithiasis, sphincter of Oddi dysfunction (SOD), and pancreas divisum, the most commonly encountered diagnoses in the work-up of ARP. Less often, pancreatic and ampullary cancers; duodenal diverticulum; pancreatic duct strictures or stones; and congenital malformations such as choledochocele, annular pancreas, and anomalous pancreaticobiliary junction may be encountered. ERCP is risky because it causes acute pancreatitis in 3% to 20% of patients17,18 depending on the indication and maneuver performed. Acute pancreatitis is more frequent when ERCP is performed for diagnostic purposes, particularly when coupled with treatment of SOD, compared with other indications, most notably bile duct stones.19 Other risk factors include multiple or high-pressure injections of contrast agent into the pancreatic duct, therapeutic intervention, a past history of pancreatitis, and operator inexperience.20 In a referral center treating 279 patients with acute pancreatitis over a 5-year period, ERCP was the causal factor in 4% of cases.21 However, 3 of 11 patients in the subgroup with ERCP-related pancreatitis died.
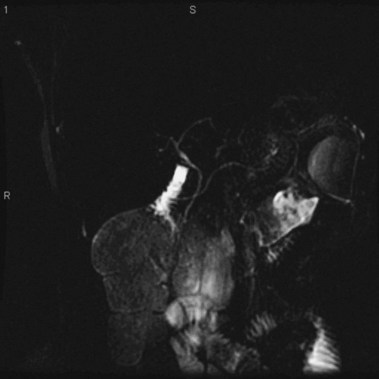
MRCP is replacing diagnostic ERCP in many centers because of ERCP-related complications.22 With heavily T2-weighted images, fluid within the bile and pancreatic ducts produces an image akin to an endoscopically generated cholangiopancreatogram. MRCP is accurate in detecting common bile duct stones23,24; its role in the evaluation of ARP includes the identification of anatomic abnormalities such as pancreas divisum (Fig. 48.1), choledochocysts, annular pancreas, and anomalous pancreaticobiliary junction.24–27 However, ERCP continues to be used in the diagnostic evaluation of ARP because of the ability to visualize the ampulla, to sample tissue and bile, and to perform sphincter of Oddi manometry.
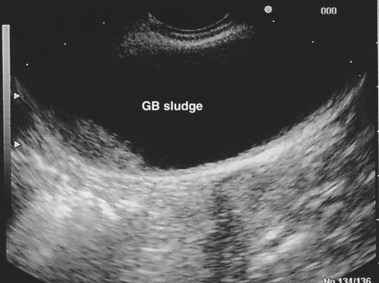
EUS uses higher frequencies than conventional abdominal ultrasound, and the image quality is not compromised by intestinal gas, providing a higher sensitivity and specificity for detecting cholelithiasis than conventional ultrasound.28–30 It has been shown to be as accurate as ERCP in the diagnosis of choledocholithiasis,31 and the positive predictive value for biliary tract disease including microlithiasis in the gallbladder and biliary sludge (Fig. 48.2)32,33 is about 98%.14 It remains the endoscopic procedure of choice for visualizing the pancreas,34–38 and it is the most accurate technique for the detection and local staging of pancreatic carcinoma.39,40 EUS is also useful in detecting changes in the pancreatic parenchyma and ducts.38,39
In patients with ARP, EUS correctly identified a cause of acute pancreatitis in 155 of 168 patients in whom a cause was found by a multidisciplinary diagnostic approach, involving ERCP, bile crystal analysis, surgery, and medical follow-up. EUS may also be useful in the detection of pancreas divisum,41 SOD,42 and anomalous pancreaticobiliary junction,43 but more data are required before the performance characteristics of EUS for these diagnoses is known. Given the high yield and lower complication rate compared with ERCP, EUS is being used earlier in the work-up of ARP. However, a consensus has not been reached so far regarding the exact place for EUS in the diagnostic algorithm for ARP. Increasingly, EUS evaluation of the pancreas, ampulla, and biliary system is being used to detect potentially diagnosable and treatable causes of ARP before use of ERCP.
Pancreas Divisum
Introduction
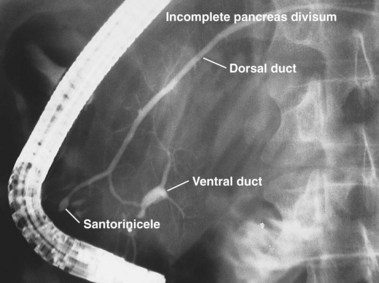
The term pancreas divisum refers to two pancreatic ductal systems that do not unite during embryologic organogenesis and drain separately via the two duodenal papillae—the dominant dorsal system through the minor papilla and the smaller ventral system through the major papilla (see Fig. 48.1). Incomplete pancreas divisum is a threadlike communication between dorsal and ventral pancreatic ducts and, when symptomatic, is treated similar to complete pancreas divisum (Fig. 48.3).
Epidemiology
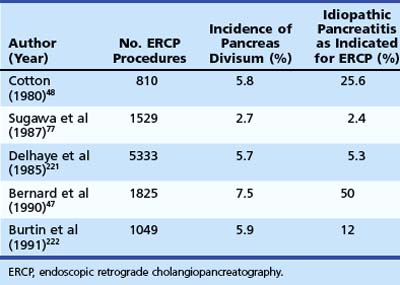
Pancreas divisum is the most common congenital anomaly of the pancreas and may be found in 7% to 14% of autopsy series.44–46 The frequency of pancreas divisum among ERCP series varies greatly (2.7% to 7.5%) and depends on the population studied and the diligence with which complete pancreatography is pursued. Pancreas divisum is reported to occur less often in Asians (1% to 2%)47 and blacks (2%).48 The clinical significance of pancreas divisum is controversial. Although estimates reveal that less than 5% of the population with pancreas divisum ever develops pancreatic symptoms, authorities recognize its association with ARP, CP, and abdominal pain.49–51 Patients undergoing pancreatography for documented pancreatitis are substantially more likely to have pancreas divisum than patients who have incidental pancreatograms during ERCP or for unexplained chronic abdominal pain.52 Table 48.2 summarizes the larger ERCP series of patients with pancreas divisum and pancreatitis. Caution should be exercised when assigning causation to pancreas divisum in patients with ARP given its prevalence in the population, and other known causes of pancreatitis should be sought for and excluded.
Pathogenesis
Because most exocrine flow is routed through the minor papilla in this ductal anomaly, it is hypothesized that in some patients an increased resistance to flow across this small orifice results in dorsal duct hypertension and clinical symptoms.53–56 The resulting increased dorsal duct pressure may also make the pancreas more prone to injury from alcohol and drugs.57,58 Surgical and endoscopic procedures aimed at decreasing resistance to flow across the minor papilla have been reported with varying success. Another mechanism by which pancreas divisum may lead to ARP is a decrease in the cystic fibrosis transmembrane conductance regulator protein (CFTR) function.59 The association between loss of CFTR function and ARP is discussed later in the section on genetic causes.
Diagnosis
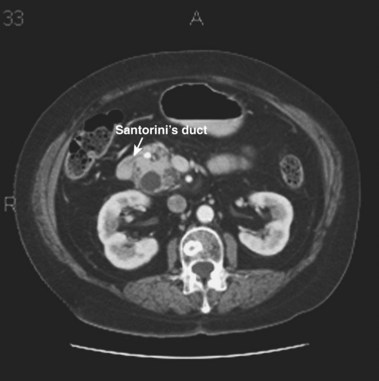
ERCP remains the “gold standard” for diagnosing pancreas divisum. A characteristic ventral pancreatogram with an attenuated duct that arborizes strongly suggests pancreas divisum. Caution must be exercised in patients with an obstructed pancreatic duct that may look like pancreas divisum. Pancreas divisum is confirmed by locating the minor papilla and injecting contrast agent into the dorsal duct (see Video). The minor papilla is usually located 2 cm proximal and 2 cm medial to the major papilla. It is best seen with the duodenoscope passed in a long position, without reducing the loop along the greater curve of the stomach. Occasionally, the minor papilla cannot be identified. The use of intravenous secretin (0.2 mcg/kg) during ERCP may stimulate the exocrine pancreas and help identify the minor papilla as a point origin of clear liquid “squirting” into the duodenum.60 The prior application of methylene blue to the periampullary mucosa before intravenous secretin has been suggested to assist in the identification of the minor papilla.61 Cannulation of the papilla may be difficult, and various tapered or metal-tipped catheters and papillotomes have been developed (Fig. 48.4). The success of minor papilla cannulation is optimized when the diagnosis is predetermined; this calls for noninvasive diagnostic modalities to avoid repeat ERCP. MRCP is best suited for this purpose. The use of intravenous secretin improves pancreatic duct visualization during MRCP.62,63 The diagnosis of pancreas divisum may be made by CT (Fig. 48.5) or EUS, but the accuracy of diagnosis of these modalities is unknown.
Clinical Presentation and Treatment
The earliest attempts at treatment for patients with presumed symptomatic pancreas divisum were surgical. The surgical procedures first performed to reduce resistance to exocrine flow consisted of a transduodenal minor and major sphincteroplasty with cholecystectomy. More recently, a transduodenal minor sphincterotomy or sphincteroplasty alone64 has evolved as the surgical treatment of choice. The clinical presentation of ARP, the presence of minor papilla stenosis either intraoperatively or by delayed clearance of dye after dorsal ductography during ERCP, and a positive ultrasound secretin test are the best predictors of outcome after surgical intervention for pancreas divisum.65
To avoid laparotomy, numerous endoscopic maneuvers have been applied for the management of symptoms related to pancreas divisum. These have included minor papilla dilation, stent placement, and sphincterotomy. The technique of minor papilla stent placement involves free selective cannulation of the dorsal duct, placement of a guidewire, and advancement of a stent specifically designed for use in the pancreas over the guidewire with a pushing cannula (see Video). These stents are plastic and vary in diameter from 3-Fr to 10-Fr (Fig. 48.6). Larger stents (7-Fr and 10-Fr) are reserved for patients with dilated pancreatic ducts or CP. Pancreatic stents 5-Fr and larger have multiple side holes to permit drainage from side branches and have external flanges or pigtails to prevent inward migration. Some stents have internal barbs to prevent outward migration. The number and need for multiple stent exchanges have not been firmly established; the interval necessary for stent exchanges likewise has not been established, although most authors advocate 4 to 8 weeks for stents 5-Fr to 7-Fr in diameter.
Minor papilla dilation may be accomplished with catheter dilators or hydrostatic balloons. Catheter dilators generally vary in diameter from 5-Fr to 10-Fr and are advanced into the dorsal ducts over a preplaced wire. Although balloons vary in diameter, for the sole purpose of dilating the minor papilla, 4-mm over-the-wire hydrostatic balloons are the smallest commercially available devices. Larger balloons should be used with caution, unless concurrent CP exists with need for additional therapy (e.g., stone extraction or stricture dilation; see Chapter 49).
Two techniques have been described for minor papilla sphincterotomy: standard traction papillotomy (see Video) and needle-knife sphincterotomy over a stent. Traction papillotomes with braided or monofilament cutting wires can be used to accomplish traction papillotomy. The papillotome tip in the pancreatic duct usually directs the wire in a 12 o’clock to 2 o’clock direction; the length of the cut depends on the size of the minor papilla mound, usually about 5 mm. Some authors advocate the use of pure cutting current to prevent cautery to the pancreatic parenchyma with resulting stenosis of the outflow tract. It is generally advisable to leave a temporary pancreatic stent, which has been shown to decrease the risk of postprocedure pancreatitis for pancreatic sphincterotomy of the major papilla.66,67 The needle-knife technique involves placing a pancreatic stent into the dorsal duct and using it as a guide to create an approximately 5-mm cut over the stent. The stent may be left in place temporarily to reduce the risk of post-ERCP pancreatitis. Minor papilla dilation alone has not been studied in a controlled fashion but has been used in combination with stent placement.
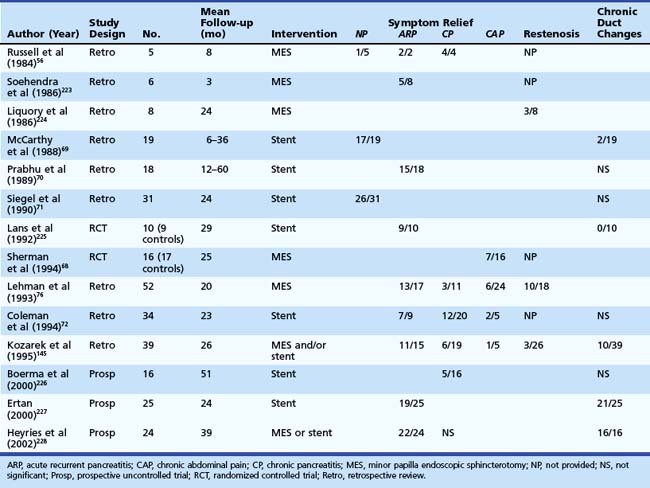
Categorizing patients with symptomatic pancreas divisum may help decide management and predict outcome (Table 48.3). Two prospective, randomized, controlled trials have evaluated endoscopic therapy for patients with pancreas divisum. One trial looked at the role of stent placement in the minor papilla in patients with pancreas divisum and ARP and reported 90% success over a mean 29-month period.64 The second trial evaluated the role of minor papillotomy in treating patients with pancreas divisum and chronic abdominal pain and reported symptomatic relief in 44% of patients.68 Studies looking at dorsal duct stent placement and minor papillotomy summarized in Table 48.369–72 suggest symptomatic improvement or resolution of ARP in 70% to 80% of patients with pancreas divisum; however, these studies are limited by heterogeneous study populations, varied follow-up, and lack of controls. The response in the setting of CP is less satisfactory and, for chronic abdominal pain, suboptimal and ill-advised in our opinion.
Regarding the choice of therapy, most authorities now favor minor papillotomy over dorsal duct stent placement. The overall success rate of endoscopic therapy (stent placement, dilation, or sphincterotomy) is similar to the results of surgical sphincteroplasty. The restenosis rate in the surgical literature appears to be less than for endoscopic minor papillotomy, although reports suggesting that patients who have restenosis after ES also have restenosis after sphincteroplasty.73 Endoscopic techniques seem preferable as a first choice because laparotomy can be avoided. The short-term success rates of minor papilla ES and surgical sphincteroplasty may be similar, but long-term follow-up and comparative trials are needed before firmer recommendations regarding procedure choice and cost-effectiveness can be made.
Complications
Complications of stent therapy include acute pancreatitis; the induction of pancreatic ductal changes, many of which may be irreversible74; stent occlusion or migration; pancreatic duct perforation; and the need for repeated procedures. Complications of minor papilla ES, including bleeding, perforation, and pancreatitis, have been reported and are similar to complications of major papilla ES.75 Lehman and coworkers76 reported a 15% procedural complication rate for minor papilla ES, primarily mild pancreatitis. Frequency of restenosis is reported to be 5% to 10%.77
Biliary Microlithiasis
Introduction
Many terms have been used interchangeably for biliary microlithiasis including biliary sludge and biliary sand. The term biliary microlithiasis typically refers to finding cholesterol monohydrate crystals and calcium bilirubinate granules on light microscopy of an endoscopically acquired centrifuged sample of bile.78 The criteria for differentiating between biliary microliths and small stones are unclear, but generally a gallstone is defined as a particle with a diameter greater than 2 to 3 mm that cannot be crushed by digital compression.78 Biliary sludge is the ultrasound finding of low-level echoes without acoustic shadowing that gravitate toward the dependent portion in the gallbladder79 and move with positioning. Biliary sludge consists of cholesterol monohydrate crystals and calcium bilirubinate granules suspended in gallbladder mucus. Other calcium salts, proteins, and xenobiotics such as ceftriaxone can also be found.80
Epidemiology
Similar to gallstones, the risk of developing biliary microlithiasis is increased in women and in several conditions including pregnancy,81,82 rapid weight loss,83 critical illness,84 prolonged fasting,85 long-term administration of total parenteral nutrition,85–88 ceftriaxone86–91 or octreotide administration,92–94 and bone marrow or solid organ transplantation.95–99 The development of ARP in these clinical situations should prompt an aggressive search for microlithiasis (approximately 31%). Approximately 31% of patients with nonalcoholic pancreatitis have biliary microlithiasis, and 74% of patients with “idiopathic” pancreatitis have been shown to have biliary microlithiasis.11,100 Two prospective studies of consecutive patients with apparently idiopathic pancreatitis found that two-thirds to three-fourths had microlithiasis as the presumed cause, as documented by biliary drainage studies, follow-up ultrasound studies, and ERCP with sphincterotomy or cholecystectomy.11,100
Pathogenesis
The clinical significance of biliary microlithiasis is very controversial. Experts remain divided over whether it is a transient phenomenon or a precursor to gallstones. After chemical dissolution of gallstones, gallbladder sludge is usually seen on ultrasound before gallstone recurrence,101 suggesting that the pathogenesis of sludge is similar to that of gallstones.102–106 Sludge resolves spontaneously in most cases, and gallstones form in only a few individuals with sludge. There are few studies of the pathogenesis and natural history of biliary sludge, and most are limited by insufficient follow-up.101,107,108 Three clinical outcomes were noted in one study, including complete resolution, a waxing and waning course, and gallstone formation.101 Sludge found in patients with abdominal pain seems to disappear spontaneously in about 50% of cases. Asymptomatic persistence is seen in about 20% of cases over 3 years, and symptoms may develop in 10% to 15% of patients. Gallstones develop in 5% to 15%.109
Further support for the role of biliary microlithiasis in producing pancreaticobiliary symptoms comes from the observations that symptomatic patients with gallstones receiving ursodeoxycholic acid had resolution of symptoms in 3 months, although the number and size of gallstones remained unchanged.110 The supposition is that the treatment resolved concurrent biliary microlithiasis, which led to the symptoms. Asymptomatic patients with gallstones who receive shock wave lithotripsy have been reported to develop biliary colic, cholecystitis, or acute pancreatitis.111–115 In this situation, the therapy may have created sludge, which produced the symptoms.
Hypothetically, microlithiasis can lead to pancreatitis through numerous mechanisms. Small stones may transiently impact at the papilla leading to pancreatic duct obstruction and pancreatitis.116 Recurrent passage of stones may lead to papillary stenosis or SOD, both of which are associated with pancreatitis.117
Diagnosis
The diagnosis of biliary sludge is confirmed by ultrasound. The sensitivity of transabdominal ultrasound for sludge is approximately 55%, whereas the sensitivity of EUS is approximately 96% (see Fig. 48.2), compared with duodenal bile collection (67%).118,119 Although clinically less applicable, microscopic examination of gallbladder contents is considered the diagnostic “gold standard” and allows the chemical composition of sludge to be defined. The sensitivity is 83% when bile is obtained directly from the common bile duct during ERCP.120 Bile sampling is indicated only if less invasive studies are negative, the clinical suspicion for microlithiasis is high, and the results would guide management. Techniques vary as to the site of bile collection, cholecystokinin use, sample processing, and criteria for a positive test. The relationship between the quantity of crystals and the clinical outcome remains unproved.
Treatment
Numerous therapeutic options are available to treat symptomatic biliary microlithiasis and include cholecystectomy, ES, and chemical dissolution. The benefit of therapy has been shown by the significant decrease in recurrent episodes of pancreatitis after therapy (<10%) versus a recurrence rate of approximately 66% to 75% in untreated patients.11,121–126
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree