Fig. 6.1
Anterior abdominal wall musculature and fascia
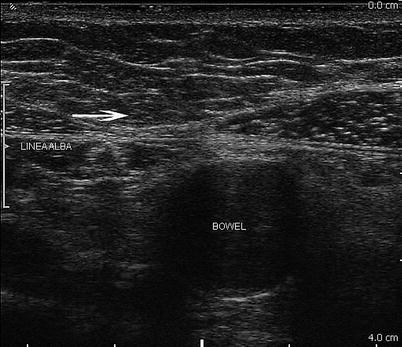
Fig. 6.2
Abdominal wall anatomy at the abdominal midline. The linea alba is noted by the arrow
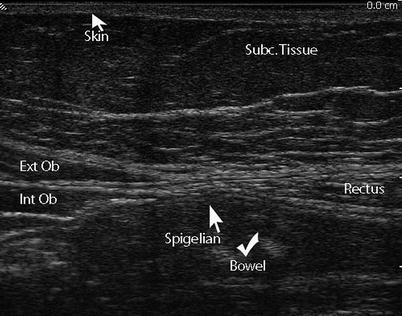
Fig. 6.3
Linea semilunaris. The linea semilunaris along the lateral margin of the inferior rectus muscle is where a Spigelian hernia is expected to occur
Table 6.1
Ultrasonographic appearance of abdominal wall anatomy
Abdominal wall component | Sonographic features |
|---|---|
Skin | Hyperechogenic |
Subcutaneous tissue | Oval hypoechoic nodules demarcated by echogenic septae |
Perforating vessels may be present | |
Fascia | Dense hyperechoic bands |
Muscle | Intermediate echogenicity, echogenic dotting within each layer |
Preperitoneal space | Hypoechoic adipose and areola tissue |
Inferior and superior epigastric vessels visible with Doppler |
On ultrasound, the skin is echogenic and measures a couple of millimeters in thickness. The subcutaneous tissue will appear as oval hypoechoic nodules demarcated by echogenic septae. Interspaced within the tissue are perforating vessels. Below the subcutaneous layer lie the muscles and their investing fascia (Fig. 6.4). Inferiorly the external oblique aponeurosis curves inward on themselves to form the inguinal ligament (Poupart’s ligament), which is the shelf of the inguinal canal. The internal oblique muscles lie posterior to the external oblique muscles. Also, in males, inferiorly, the internal oblique aponeurosis fibers run alongside the spermatic cord to form the cremasteric muscles. The transversalis fascia lies posterior to the transversalis muscle and anteromedially forms the most posterior layer of the posterior rectus sheath prior to inserting at the linea alba. Medially, between the linea semilunaris and the linea alba lie the rectus abdominis muscles (Fig. 6.2). On ultrasound, the above muscle layers are of intermediate echogenicity and have echogenic dotting within each layer. The fascial layers separating the muscle layers appear as echogenic bands (Fig. 6.4).
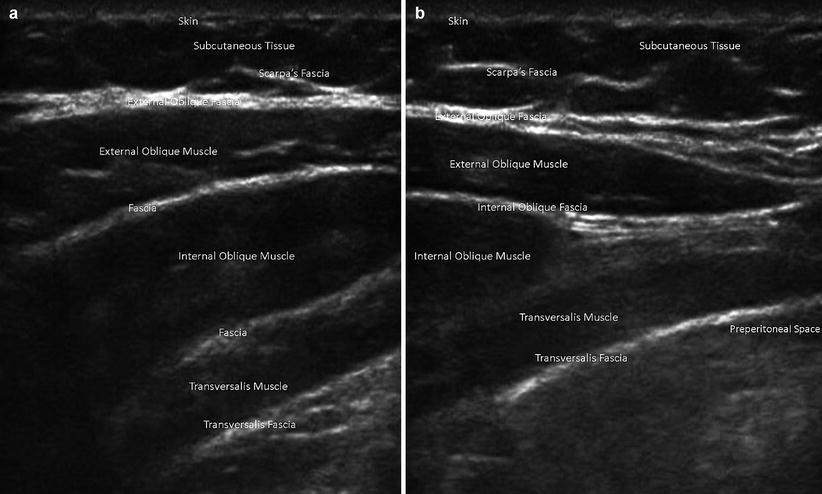

Fig. 6.4
Abdominal wall muscles and fascia on ultrasound. (a) Lateral abdominal wall. (b) Medial abdominal wall, lateral to linea semilunaris
Preperitoneal Space and Peritoneum
The preperitoneal space lies deep to the abdominal wall muscle layers and the transversalis fascia. This space contains adipose and areolar tissue and includes the inferior and superior epigastric artery and vein and the umbilical ligaments. The medial umbilical ligament is the obliterated remnant of the fetal umbilical artery, and the median umbilical ligament is the remnant of the urachus, which persists as fibrous cord along the midline extending from the bladder to the umbilicus. Superior to the umbilicus and extending towards the liver is the falciform ligament. The ligamentum teres, also known as the round ligament, is the free margin of this falciform ligament and is the obliterated remnant of the umbilical vein coursing from the left portal vein to the umbilicus. The preperitoneal fat layer is a relatively thin layer measuring less than a cm in thickness on ultrasound.
Vascular Supply of the Anterior Abdominal Wall
The inferior intercostal, lumbar, epigastric, and deep circumflex iliac arteries supply the arterial blood supply to the abdominal wall. The intercostal and lumbar arteries course alongside the nerves as bundles between the internal oblique and transversus abdominis muscles (Fig. 6.3). The superior epigastric artery is one of the terminal branches of the internal mammary artery and courses within the rectus sheath where it collateralizes with the inferior epigastric artery, which is a branch of the external iliac artery that courses superiorly in the preperitoneal space before piercing the rectus sheath. When imaging the abdominal wall with ultrasonography, it is important to identify the vessels in this preperitoneal layer prior to proceeding with any invasive intervention (Fig. 6.5a, b). The lymphatic drainage of abdominal wall is also similar to the venous outflow system whereby the supraumbilical lymph channels ultimately drain into the axillary basin while the infraumbilical channels drain into the superficial inguinal nodes. The lymphatic vessels from the liver also communicate with the periumbilical lymphatics via the ligamentum teres.
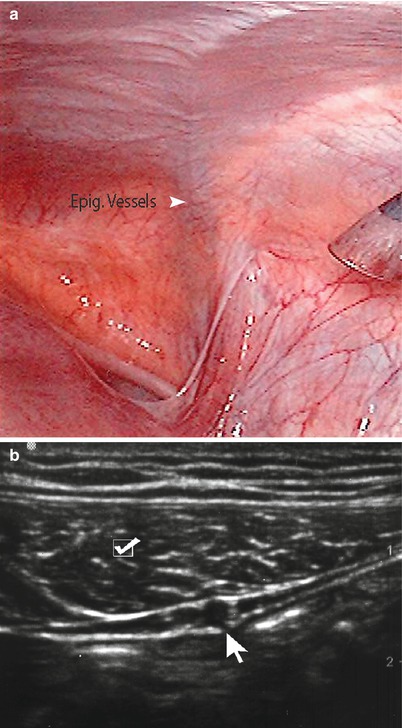

Fig. 6.5
(a) Note epigastric vessels through laparoscopic view. (b) Ultrasound imaging of epigastric vessels (arrow) and rectus abdominis muscle (checkmark)
Abdominal Wall Pathology and Intervention
A thorough physical examination will identify most pathological conditions within the abdominal wall. However, patient body habitus, edema, or complex masses can present a diagnostic dilemma for the clinician. US serves as a first-line tool in the identification, characterization, and management of abdominal wall pathology. Most pathology of the abdominal wall presents as a mass that may or may not be associated with skin changes. Ultrasound can further discern characteristics of the mass (solid versus cystic, complex versus simple, border regularity, etc.) as well as its location to the abdominal cavity and relationship to other abdominal wall structures. It is especially valuable in difficult-to-examine patients that are obese or comatose (Fig. 6.6). An image-guided biopsy using a #22 gauge needle or a #14 core biopsy can accurately and safely make a diagnosis after initial physical examination and ultrasound imaging [6, 7].
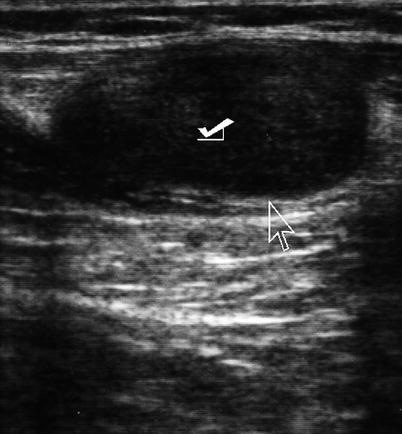

Fig. 6.6
Ultrasound of the abdominal wall depicting tumor implant (check mark). Arrow points to interface between tumor and wall
In this section, we review abdominal wall pathology and discuss US-guided interventions (Table 6.2).
Table 6.2
Ultrasonographic appearance of abdominal wall masses
Abdominal wall pathology | Sonographic features |
|---|---|
Rectus sheath hematoma | Primary hypoechoic but varies with age and location of the hematoma |
Acute hematomas are homogenous and echogenic while late-stage hematomas can be anechoic | |
Above the arcuate line: lens-shaped appearance | |
Below the arcuate line: more extensive and may cross midline | |
Hematoma | Variable echogenicity |
Acutely hyperechoic | |
Seroma/cyst | Simple fluid: anechoic, homogeneous appearance, posterior enhancement |
Complex: anechoic and hyper-/hypoechoic, heterogeneous | |
Abscess | Echogenic rim enhancement |
Variable echogenicity within cavity with presence of debris and septations | |
Urachal cyst | Well-circumscribed fluid collections |
Located along the lower midline | |
Anechoic | |
Varying internal echogenicity when infected with associated adjacent soft-tissue stranding | |
Abdominal wall varices | Dilated veins visible with Doppler |
Recanalized umbilical vein is anechoic in background of fatty falciform ligament | |
Endometriosis | Irregular hypoechoic mass |
Scattered internal echoes and internal vascularity |
Rectus Diastasis
Rectus diastasis is characterized by a thinning of the linea alba so that the distance between the rectus muscles is increased. There is no defect in the underlying aponeurosis and transversalis fascia; thus, there is no actual hernia. The patient may have a midline bulge as a result of this diastasis that is often mistaken as a hernia (Fig. 6.2). Management usually entails reassurance to the patient. If surgical correction is desired by the patient, abdominoplasty techniques are utilized. Ultrasound has been shown to be an accurate method of measuring the supraumbilical and periumbilical diastasis for the purposes of operative planning [8].
Rectus Sheath Hematomas
Hematomas within the rectus sheath are uncommon. They may develop spontaneously, yet, they often are associated with traumatic injury, pregnancy, coughing, or the use of anticoagulation therapy. They present with an acute onset of abdominal pain and tenderness that may be mistaken for peritonitis. In advanced cases, there may also be periumbilical or flank ecchymosis. Abdominal ultrasonography and CT scan aid in identifying the extent of a hematoma. The sonographic appearance of a rectus sheath hematoma varies with the age and location of the hematoma (Fig. 6.7). Above the arcuate line, the hematoma will have a lens-shaped appearance, whereas below the arcuate line, it may be more extensive, even crossing the midline or compressing the bladder [1]. Hematomas are primarily hypoechoic but can have septal or cystic components with echogenic borders [9]. Varying echogenicities within the hematoma represent different states of organized clot formation [10, 11]. Acute hematomas are homogenous and echogenic, while late-stage hematomas can be anechoic [10].
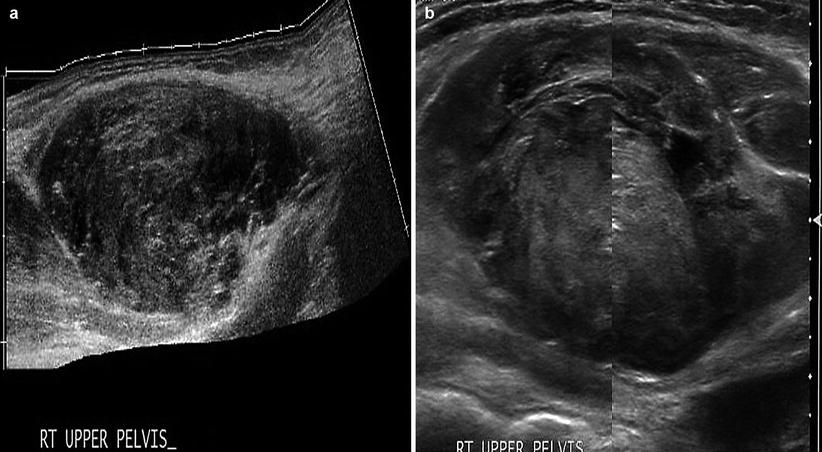

Fig. 6.7
Rectus sheath hematoma. (a) Rectus sheath hematoma below the arcuate line, with varying stages of echogenicity. (b) Acute-stage rectus sheath hematoma, with early organized clot formation (hyperechoic)
Management is usually conservative involving bed rest, analgesics, correction of coagulopathies, and blood transfusions as needed. If persistent bleeding is suspected, angiographic embolization of the bleeding of the vessel may be warranted, and if this is not available or successful, operative hemostasis and hematoma evacuation may be necessitated. Ultrasonography can be utilized to screen for hematomas that may require surgical intervention. Hematomas that are larger in diameter and demonstrate the presence of intra-abdominal free fluid on ultrasound are more likely to benefit from surgical exploration [12]. Ultrasonographic treatment of rectus sheath hematomas, where repeated sessions of nonthermal pulsed sonography are employed, has also been reported in the physical therapy literature [13].
Abdominal Wall Fluid Collections
Abdominal wall fluid collections include cysts, hematomas, seromas, and abscesses. Ultrasonography is a useful modality in identifying a fluid collection and its characteristics. Hematomas may be spontaneous, postsurgical, or related to anticoagulation therapy or traumatic injury. If warranted, hematomas may be drained with image guidance. Abscesses have a variable appearance on ultrasound. They are typically irregular fluid collections containing septations and fluid debris and may have air-fluid levels (Fig. 6.8). They may also exhibit peripheral hyperemia with Doppler evaluation [10]. If gas bubbles are present with the abscess, they will be echogenic and demonstrate acoustic shadowing [14]. Seromas are usually more homogenous anechoic or hypoechoic fluid collections on ultrasound (Fig. 6.9). They are usually encountered following an abdominal operation, particularly following ventral hernia repairs. The development of postoperative seromas may be prevented with the use of pressure dressings and abdominal binders. If there is no evidence of superinfection, it is acceptable to observe a seroma without intervention, as there is a risk of introducing infection with needle aspiration. If a seroma or hematoma appears infected or an abdominal wall abscess is identified, operative drainage or needle drainage with image guidance is warranted in addition to antibiotic therapy. For larger fluid collections that are percutaneously drained, a drain may be left in place to facilitate additional drainage (Figs. 6.10 and 6.11).
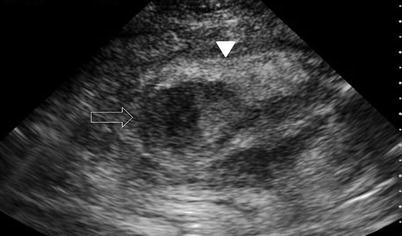
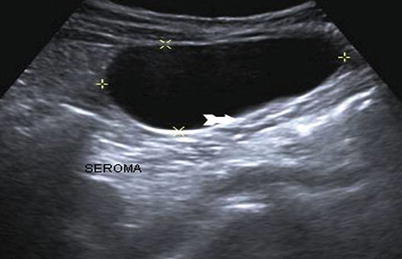
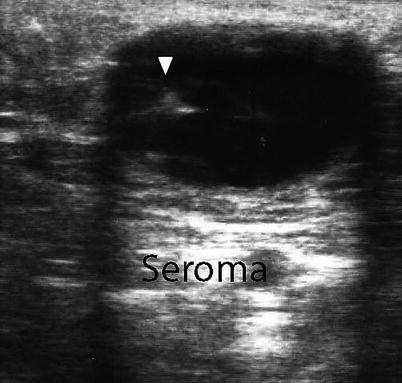
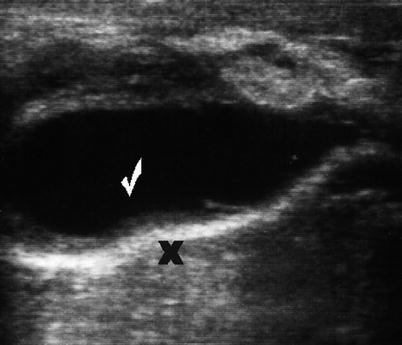

Fig. 6.8
Abdominal wall abscess (solid arrow). Abscess at umbilical incision site following laparoscopic appendectomy. Echogenic rim enhancement is seen. The abscess cavity demonstrates varying echogenicities with debris

Fig. 6.9
Postoperative seroma. Anechoic fluid collection near umbilical incision consistent with postoperative seroma

Fig. 6.10
Postoperative abdominal wall mass representing a seroma with enhancement. Note needle within the seroma (arrowhead)

Fig. 6.11
Postoperative seroma (checkmark) following repair of ventral hernia. Note mesh (X)
Abdominal Wall Neoplasms
Abdominal wall neoplasms present as painless palpable masses. Primary lesions may arise from any of the abdominal wall components: connective tissue, muscle, fat, blood vessels, or lymphoid tissue. These include benign soft-tissue neoplasms such as lipomas and desmoid tumors as well as malignant neoplasms such as sarcomas. Desmoid tumors and sarcomas are the most common primary malignancies of the abdominal wall. Metastatic neoplastic lesions may also be found in the abdominal wall, which often is associated with transperitoneal seeding of the abdominal wall by intra-abdominal malignancies (Fig. 6.12). Ultrasound examination permits precise definition of the mass as well as accurate biopsy. A diagnostic dilemma frequently occurs with these incisional wall masses where a recurrent tumor implant, a hernia, or a fluid collection could be found (Fig. 6.13). Ultrasound guidance is of value in obtaining a diagnostic biopsy of small nodules in the abdominal wall that are concerning for metastases (Table 6.3).
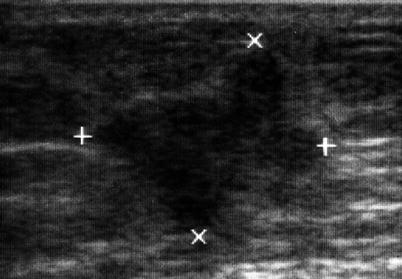

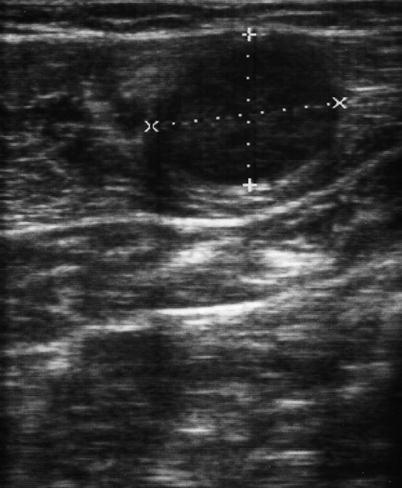
Fig. 6.12
Metastatic nodule in the abdominal wall following a resection for retroperitoneal sarcoma. The axis of the tumor (+, ×)

Fig. 6.13
Postoperative incisional hernia with incarcerated bowel
Table 6.3
Ultrasonographic appearance of abdominal wall tumors
Abdominal wall pathology | Sonographic features |
|---|---|
Lipoma | Variable echogenicity that is discrete from the surrounding fat and muscle |
May have an echogenic capsule | |
Hemangioma | Multiple hypoechoic or anechoic cystic areas within an echogenic hypervascular background |
Desmoid tumor | Solid |
Hypoechoic | |
Abutting fascial planes or muscular tissue | |
Sarcomas | Irregular |
Solid | |
Hypoechoic | |
May have localized areas of necrosis or fluid | |
Metastatic lesions | Irregular |
Hyperechoic | |
Irregular shadow |
Benign Tumors
Lipomas, neurofibromas, and hemangiomas are the most common benign neoplasms of the abdominal wall. Lipomas are well-circumscribed mobile lesions. On ultrasound, they will have a variable echogenicity that is discrete from the surrounding fat and muscle, and they may have an echogenic capsule [15] (Fig. 6.14). Hemangiomas are typically very small in diameter (millimeters) and on ultrasound will have multiple hypoechoic or anechoic cystic areas within an echogenic hypervascular background [10].
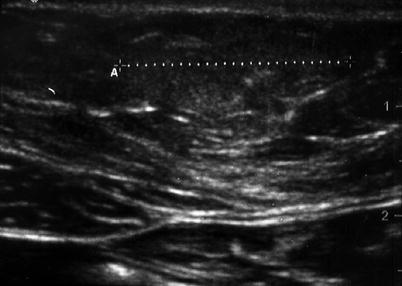

Fig. 6.14
Subcutaneous abdominal wall lipoma. Notice the smooth border and isoechoic content. Lipomas could present with bilateral or unilateral shadows
Desmoid Tumor
Desmoid tumors, or aggressive fibromatosis, are rare neoplasms that arise from fibroblast cells in either fascia or muscle. They may be intra-abdominal (pelvic and mesenteric), extra-abdominal (shoulder girdle or extremities), or abdominal wall tumors. When the tumor is superficial, it arises from the fascia and typically is slow growing in nature and of small size. These lesions are commonly referred to as Dupuytren’s fibromatosis. Deeper lesions arise from the musculoaponeurotic tissues and are usually more aggressive in growth rate and size. Although these tumors do not metastasize and are thus considered benign lesions, they are locally aggressive and often recur following resection.
Additional characteristics of the mass and the extent of involvement may be delineated with the use of an ultrasound. If the lesion is solid, hypoechoic, and abutting fascial planes or muscular tissue, one should be suspicious for a desmoid lesion. If an ultrasound is inadequate, further extent of the lesion may be defined with the use of MRI. The lesion requires biopsy for diagnosis, which may be obtained with a core needle or as an incisional biopsy under ultrasound guidance.
The treatment of abdominal wall desmoids involves surgical resection with tumor-free margins. Local recurrence rate of these tumors can be as high as 40 % [16], and these recurrent tumors will also require resection. If a lesion is unresectable, primary radiation treatment may be considered as well as palliative chemotherapy with antiproliferative cytotoxic agents.
Sarcoma
The most common primary malignant neoplasm involving the abdominal wall is a sarcoma. These sarcomas, depending on what layers and cell types of the abdominal wall soft tissue are involved, can be of several subtypes: liposarcoma, fibrosarcoma, leiomyosarcoma, rhabdomyosarcoma, and malignant fibrous histiocytoma. The clinical progression of these tumors is reflective of their histology, size, and location.
These abdominal wall sarcomas can present as painless abdominal wall masses. Suspicion for malignancy arises with masses that are solid, large, fixated, and fast growing. On ultrasound, a sarcomatous lesion will be a hypoechoic solid lesion that may have localized areas of necrosis or fluid [10]. MRI can also be used to further delineate extent of disease. These lesions require guided biopsies, either incisional or percutaneous, to confirm their pathological diagnosis.
Metastatic Neoplasm
Transperitoneal, hematogenous, lymphatic seeding of intra-abdominal carcinomas or melanomas may result in metastatic lesions in the abdominal wall (Fig. 6.15). Cases of port-site seeding after laparoscopic surgery have also been reported in the literature, particularly following laparoscopic cholecystectomy where the cancer diagnosis was unknown at the time of operation. A metastatic deposit at the umbilicus is known as a Sister Mary Joseph’s nodule. These lesions may be characterized and biopsied with sonographic guidance and are typically treated with surgical resection and radiation as needed. Ultrasound has also been reported to be of use in guiding insertion of applicator needles that can administer brachytherapy to abdominal wall metastases from colorectal cancer [17].
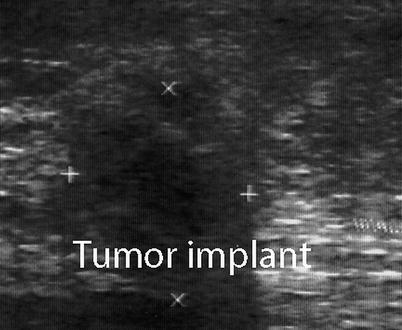

Fig. 6.15
Abdominal wall tumor implant in a patient with a history of colon cancer. The axis of the tumor (+, ×)
Other Abdominal Wall Masses
Urachal Cyst
An urachal cyst is a sinus remnant that persists between the umbilicus and bladder. It is usually present in the lower third of the urachus but may lie inferior to the umbilicus as well. These cysts can be depicted on ultrasound, where there will be well-circumscribed fluid collections that are anechoic, along the lower midline [15]. When infected, they have varying internal echoes and may be associated with adjacent soft-tissue inflammatory changes and urinary bladder wall thickening on ultrasound [18]. If symptomatic or associated with recurrent urinary tracts infections, these cysts should be surgically excised (Fig. 6.16).
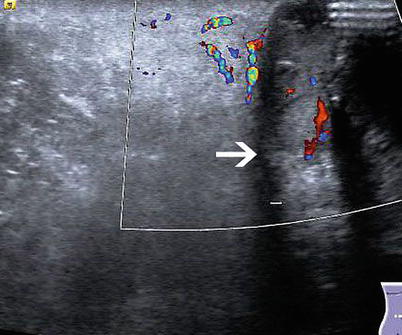

Fig. 6.16
Infected infraumbilical abdominal wall urachal cyst (arrow points at cyst wall)
Vascular Anomalies
Patients with portal hypertension often recannulate their umbilical vein as a means to shunt blood flow to the systemic veins (caput medusae). Ultrasound with color Doppler can be utilized as a means to avoid these varices when performing abdominal wall procedures such as a paracentesis or placement of a percutaneous endoscopic gastrotomy tube in patients with portal hypertension (e.g., cirrhotic patients) [19].
Scar-Related Masses
Common masses that present in relation to prior surgical scars include stitch granulomas, heterotropic calcifications, and endometrial implants. A stitch granuloma will occur near a retained nonabsorbable suture and will demonstrate irregular borders and heterogenicity on ultrasound. Heterotrophic calcifications are benign lesions, which on US examination have posterior acoustic shadowing [10]. Endometriosis of the abdominal wall may occur following a prior gynecologic operation. It is the most common site of extraovarian or extrauterine endometriosis following a cesarean section operation [20]. The lesion may present as a painful solid mass near a previous scar. On ultrasonography, it appears as a hypoechoic solid lesion, with scattered internal echoes and internal vascularity that can be demonstrated with color Doppler [21] (Fig. 6.17).