Fig. 11.1
International Prostate Symptom Score (IPSS) questionnaire
Changes in subjective parameters have been shown to correlate with changes in objective parameters after brachytherapy [2]. Maximum flow rate, voided volume, and post-void residual urine volume are decreased at 1 and 6 months after implantation and return to baseline by 1 year. Prostate volume as measured on transrectal ultrasound has been shown to decrease at a year after brachytherapy, although this change is not seen in patients treated with neoadjuvant hormones [2]. Dysuria has been shown to peak at 1 month after brachytherapy. In a series of 581 patients with preimplantation alpha blocker therapy, the frequency and severity of dysuria were found to improve steadily over time with near complete resolution at 45 months [3]. Of the 7 IPSS questions, nocturia and incomplete voiding were found to be the best surrogates for dysuria. Neither clinical nor implant related factors were predictive of dysuria. In a study of 1932 patients treated with brachytherapy alone or with external beam radiotherapy, at 10 years after brachytherapy, minimal change was seen in the American Urological Association Symptoms Score (AUASS), a questionnaire similar to the IPSS. Patients presenting with high initial scores had the greatest improvement from baseline (Fig. 11.2) [4]. Biological effective dose, external beam radiotherapy, hormonal therapy, isotope, patient age, and prostate size were not found to affect long-term urinary symptoms.
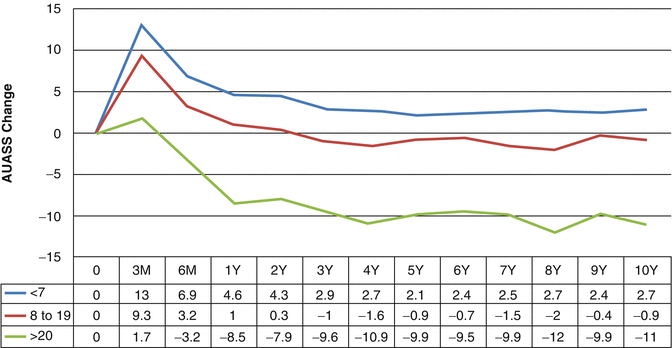

Fig. 11.2
Change in AUASS over time by pre-implant severity category (p = 0.001 for all points). Used with permission [4]
Mr. L had an IPSS score of 18, a maximum flow rate of 8, and a post-void residual (PVR) of 120 mL. He was counseled that his symptoms would likely improve with time and agreed to observation. At 6 months after brachytherapy he had an IPSS score of 8 and at a year after brachytherapy, his IPSS returned to his baseline of 3.
Case 2 (This Patient Is 2 Years Post Brachytherapy/EBRT)
Mr. B is a 68-year-old man who was treated with brachytherapy and external beam radiotherapy for Gleason 4 + 4 = 8. He initially had significant dysuria which was managed conservatively and resolved. Two years after completion of brachytherapy his symptoms returned.
Patients treated by brachytherapy have more symptoms soon after the procedure than those treated by external beam irradiation (EBRT) secondary to the trauma of the needle punctures. Early urinary morbidity does not necessarily predict worsened long term urinary function [5]. Urinary morbidity peaks immediately following radiation and then slowly returns to baseline. In a cohort of 1932 men treated with prostate brachytherapy alone or with external beam radiation followed a mean of 6.8 years, AUASS peaked at 0–3 months after brachytherapy implant. This was followed by a steady return to within 1 point of baseline by 3 years (Fig. 11.2) [4]. This study also found that patients presenting with high initial symptom scores had the greatest improvement with an average 11-point reduction in symptoms at 10 years. While many patients have resolution of symptoms after the initial peak, a late worsening or “flare” in symptoms has also been described. This transient late exacerbation of urinary symptoms occurs in over a third of patients by 5 years, most commonly 2 years after treatment. Flare has been defined as a rise in IPSS from the nadir symptom score of at least five points. Neither clinical nor implant related factors have been shown to be predictive of flare [6]. A “PSA bounce” has also not been found to be associated with urinary symptom flare. The patient’s symptoms resolved over the following 3–6 months.
Case 3 (This Patient with a Large Prostate is Being Counseled about Brachytherapy)
Mr. P is a 69-year-old man who presented to his urologist with LUTS. The urologist performed a digital rectal exam and found a large prostate approximately 60 g with a nodule suspicious for cancer. Mr. P underwent biopsy which showed Gleason 3 + 4 = 7 with a prostate volume of 60 cc. He was interested in radiation therapy and was curious to know how it might affect his urinary symptoms and quality of life.
Patients receiving radiation therapy may experience urinary symptoms resulting from patient or treatment specific factors. Patient specific factors which can affect voiding symptoms include pretreatment symptom score, androgen deprivation, prostate volume and transition zone index. Pretreatment IPSS has been shown to be predictive of posttreatment symptoms in a number of studies [6]. Neoadjuvant-hormonal therapy is associated with decreased rate of retention in patients with large prostate glands and IPSS greater than 15 [7]. Prostate volume has also been shown to be predictive of symptoms and hormonal therapy can affect prostate volume [8]. The transition zone index (TZI) is calculated as transition zone volume/prostate gland volume. Transition zone volume may affect LUTS; the TZI has been shown to be associated with urinary symptoms [9].
The most fundamental treatment specific factor is type of radiation. Lower urinary tract symptoms can occur after external beam radiotherapy (EBRT) as well as after brachytherapy. Zelefsky et al. compared men who were treated with EBRT with men who were treated with brachytherapy [10]. In the short term, those who received brachytherapy had slightly higher urinary toxicity (e.g., urethral stricture) compared with those who were treated with EBRT, although urinary symptoms subsequently resolved or improved in most patients. A study of the CaPSURE database, looking at locally advanced prostate cancer found no treatment modality was superior to others based on quality of life outcomes [11].
For patients undergoing brachytherapy there are a number of procedure specific factors that can affect urinary function including isotope type, delivery approach, total prostate and urethral dose, and number of needles and seeds implanted. The two commonly used isotopes for permanent prostate seed brachytherapy are iodine-125 (125I) and palladium-103 (103Pd). 125I has a half-life of 60 days with an average energy of 28 KeV. 103Pd has a half-life of 17 days with an average energy of 21 KeV. There is a theoretical concern that the rapid dose delivery associated with 103Pd could cause increased morbidity in large prostate glands. However in a study of almost 1000 patients, regardless of prostate size, isotope (125I versus 103Pd) did not impact IPSS resolution, catheter dependency, or the need for surgical intervention. Cesium-131, a newer isotope, has a half-life of 9.7 days with an energy of 29 KeV. Cesium-131 delivers 90 % of its therapeutic dose within 1 month. Because it was approved in 2003, there are no long-term studies of the urinary effects of this isotope, but theoretically the faster dose rate could result in a faster resolution of urinary symptoms [12].
There are two main approaches to delivery of prostate brachytherapy: preplanning, in which all calculations are made ahead of the implant date and preloaded needles are used, and intraoperative real-time dose calculation. Intraoperative dose calculation has been shown to result in better gland isodose coverage but is associated with higher IPSS scores after implantation which remain elevated for longer [13]. Total dose and dose-volume factors have also been shown to be correlated with IPSS and within the IPSS, specifically with frequency [14]. Dose to the urethra was found to affect frequency most significantly. The number of seeds inserted has been shown to correlate with increased need for catheterization, although it was not found to be predictive of elevated IPSS [8]. Difficulty of implant, as measured by the number of needles which had to unexpectedly be placed in unassigned coordinates, has also been shown to be associated with need for catheterization, but not with high posttreatment IPSS [8].
Given that the majority of patients with prostate cancer do not die of the disease and there are a number of treatment options for prostate cancer, quality of life after treatment can affect patient decision-making regarding treatment options. In a study looking at the early posttreatment period using the Short Form (36) Health Survey (SF-36), the best general physical functioning was reported by patients who underwent brachytherapy followed by external beam radiation and radical prostatectomy (RP) [15]. With time, quality of life differences between groups lessened. At an average of 7.5 months after treatment the general health related quality of life of patients undergoing brachytherapy with and without pretreatment external beam radiation was similar to age matched controls [16]. At a mean follow-up of 66.3 months after brachytherapy, patients were found to have urinary quality of life scores similar to newly diagnosed prostate cancer patients of comparable demographics [17]. Use of neoadjuvant or adjuvant hormonal therapy has been shown to have no significant impact on quality of life with no difference in the irritative or obstructive subscales of the AUASS at 24 months after implantation [18].
Case 4 (This Patient Is Being Counseled about Brachytherapy)
Mr. F is a 66-year-old man diagnosed with Gleason 3 + 3 = 6. He is interested in brachytherapy but is concerned about developing LUTS. He wants to know if there is anything he can do to prevent the development of these symptoms.
In many reported series, an alpha blocker is initiated before brachytherapy [3]. Alpha-blocker therapy has been shown to affect temporal resolution of urinary morbidity following prostate brachytherapy [19]. A double-blind, placebo-controlled, randomized trial found prophylactic tamsulosin (0.8 mg/day) did not significantly affect urinary retention rates, but had a positive effect on urinary morbidity at week 5 after brachytherapy implant as measured by AUASS [19]. The use of prophylactic alpha blocker therapy was examined in 234 patients including 142 who received therapy prior to implantation and until the return to baseline IPSS levels, as well as 92 who received alpha-blocker therapy only for the occurrence of obstructive urinary symptoms. While patients receiving prophylactic alpha blockers experienced a faster return to baseline IPSS levels, there was no observed difference in the incidence of urinary retention [20].
Case 5 (This Patient with a Prior TURP is 2 Years Post-brachytherapy)
Mr. W is a 71-year-old male with history of clinical T2a, Gleason 3 + 3 = 6 prostate cancer, pretreatment PSA 3.9 ng/mL, who received treatment with 125 I monotherapy 2 years ago. Two years prior to implantation he had a transurethral resection of the prostate with 20 g of tissue resected. His PSA is still decreasing at 1.5 ng/mL, but he has experienced progressive weakening of his urinary stream. His IPSS is 20 and his PVR is 100 mL. A cystoscopy reveals a circumferential proximal (prostatic) urethral stricture.
The occurrence of urethral stricture disease following brachytherapy has been reported in the range of 0–12 % [21–24]. Merrick and colleagues reported a 5.3 % 5-year actuarial urethral stricture rate, which occurred at a median time of 26.6 months [25]. A similar incidence of scarring has been noted following high dose rate brachytherapy, occurring most commonly in the bulbomembranous urethra [26]. Jarosek et al. compared long-term risks of adverse urinary events following prostate cancer treatment, including 14,259 patients with brachytherapy monotherapy using the Surveillance, Epidemiology and End Results (SEER)-Medicare database . The 10-year propensity-weighted cumulative incidence of urethral stricture or bladder neck contracture was 11.9 % (95 % CI 11–12.95) among patients treated with brachytherapy alone, compared with 19.4 % (95 % CI 18.23–20.62) for combination brachytherapy and external beam radiotherapy, as well as 19.3 % (95 % CI 18.74–19.96) for radical prostatectomy [27]. Similarly, a Cochrane systematic database review identified a pooled incidence of stricture in 2/85 (2.4 %) compared with 6/89 (6.7 %) of patients treated with prostatectomy (p = 0.221) [28].
The appearance of urethral stricture following implantation appears to be influenced by several dosimetric factors including the dose delivered to the membranous urethra. In one study of 1186 patients treated with brachytherapy, 29 developed urethral strictures, this adverse outcome was associated with higher dose to the bulbomembranous urethra (105.6 % versus 85.5 % of planned dose, p = 0.002) [29]. Patients with radiation induced strictures have been conventionally approached with initial dilation and/or endoscopic urethrotomy. Reconstruction with urethroplasty may be performed for post-radiation strictures with and without tissue transfer. Glass et al. reported on 29 patients with radiation-induced stricture in the bulbous (41 %) and membranous (41 %) urethra using anastomotic, buccal mucosal graft and perineal flap techniques with an overall success rate of 90 % [30]. In a multi-institutional review of 72 cases from 3 academic sites treated primarily with anastomotic urethroplasty, successful reconstruction was achieved in 69.7 %, and incontinence developed in 18.5 %, and was associated with longer stricture length (>2 cm) [31].
There is a distinct difference between post-TURP prostatic urethral strictures and those occurring in the membranous or bulbar urethra. In the former, extra care needs to be taken not to place seeds too close to the TUR defect or bladder neck, while still maintaining adequate dose distribution. In the latter, stricture occurs because of errant seed placement inferior to the prostate apex. This occurs when physicians do not use real-time placement and overcompensated apical coverage.
Mr. W had an incision of the stricture and was placed on CIC daily. Over a 3 month period the frequency of CIC was reduced with complete resolution of symptoms and residual urine. Patients experiencing prostatic urethral strictures with a history of TURP after brachytherapy often redeveloped their strictures following urethrotomy. This is a result of the compromised blood supply from both procedures. A proactive course combining urethrotomy with CIC has been found to be most effective at long term resolution.
Hematuria Following Radiation
Case 1 (This Patient Is 3 Years Post Brachytherapy with Microhematuria)
Mr. O is a 67-year-old male with history of clinical T1c, Gleason 3 + 4 = 7 prostate cancer (2 out of 12 cores, 15 % greatest volume in single core), pretreatment PSA 5.3 ng/mL status post 125 I monotherapy 3 years ago. He takes tamsulosin 0.4 mg daily for mildly bothersome LUTS (IPSS 9), and urinalysis sent on workup for urinary symptoms demonstrates 5–10 red blood cells per high power microscopy field (RBC/hpf) without casts, nitrites or leukocyte esterase positivity. He denies gross hematuria. A urine culture is negative. His most recent PSA is 0.4 ng/mL, and digital rectal examination is unremarkable.
Gross hematuria occurring following an interval of normal function post-implantation is a clinically significant event that warrants thorough evaluation. Therefore, most authors agree that patients with any degree of hematuria following implantation should proceed with complete hematuria evaluation. With longitudinal follow, the appreciation for delayed hematuria—both gross and microscopic among men treated with prostate brachytherapy has been described. Hematuria following implantation can be classified by temporal onset: bleeding occurring in the immediate peri-procedural period, and that occurring following a period of normal urinary function (late hematuria). The concern for secondary in-field pelvic malignancy—particularly bladder—following prostate radiation weighs considerably on the occurrence of late hematuria [32].
Reflecting long-standing expert opinion, the American Urological Association practice statement defines microscopic hematuria as three or greater red blood cells seen per high power microscopy field in the absence of a benign cause [33]. Following the immediate post-implantation period, however, it is widely regarded that new onset hematuria should not be merely attributed to an antecedent history of brachytherapy. According to the Radiation Therapy Oncology Group (RTOG), acute gross hematuria with or without clot passage is classified as a grade III toxicity, while hematuria requiring transfusion or bladder obstruction is regarded as grade IV. Similarly, cooperative group common toxicity criteria define microscopic hematuria as a grade I toxicity; gross hematuria without clots as grade II, hematuria with clots as grade III, and hematuria requiring transfusion as grade IV [34].
Barker and colleagues reported on their series from the University of Washington including 215 patients treated with permanent prostate interstitial brachytherapy (103Pd or 125I isotopes) followed for a median of 20 months [35]. Twenty-seven patients were identified (13 %) who experienced gross hematuria occurring 1 week post-implantation. Eleven patients reported one single isolated episode, while the remaining 16 reported multiple episodes. Other publications with longitudinal follow have identified bleeding occurring at later interval. Anderson et al. reported on 263 consecutive patients who received low dose rate (LDR) brachytherapy monotherapy and minimum of 1 year follow-up between 1998 and 2006 at the MD Anderson Cancer Center, noting hematuria in 13 (10 grade I, 3 grade II) [36]. Larger series with longitudinal follow have similarly identified significant gross hematuria (RTOG ≥ 3) as a rare complication. Keyes et al. reported on 712 consecutive patients receiving permanent prostate brachytherapy between 1998 and 2003 with median follow of 57 months, noting severe hematuria in 0.1 % [37, 38]. However the definition employed in this series addresses only late RTOG grade 3 hematuria, and does not capture the number of patients experiencing lesser degrees of late gross or microscopic hematuria.
We recently published our clinical experience and evaluation of patients receiving cystoscopy for gross or microscopic hematuria. Of 2532 patients, 185 (7.3 %) underwent cystoscopy for hematuria at a median time of 2.7 years following implantation [1]. Most patients presenting with gross or microscopic hematuria had no identifiable pathology on cystoscopy (118, 63 %). The most common findings included bladder tumors in 18 (9.7 %), radiation cystitis in 13 (7 %), and hypervascularity or telangiectasias of the bladder in 18 (9.7 %). Our group has previously reported on a cohort of 2454 men treated at the Mount Sinai Hospital with 5.9 year median follow-up. We identified 218 (8.9 %) who experienced gross hematuria at a median time of 2.1 years, which reflects the latency with which delayed hematuria can be observed [39].
It is not clear whether the incidence of hematuria following brachytherapy implantation is significantly different from observational studies of individuals participating in health screening, which has been cited between 2 and 31 % [33, 40–42]. There is a considerable investigational interest in the factors that predict biological response to radiotherapy, and as a corollary, the factors which predispose to the development of treatment related toxicities. In their 2003 study, Barker et al. reported that hematuria was not associated with patient age (p = 0.4), pre-implant prostate volume (p = 0.46), pre-implant American Urological Association symptom score (p = 0.66), or maximal or mean urethral dose [35]. However, among our cohort, the development of late gross hematuria was significantly associated with larger prostate size (>40 cm3), external beam radiation, and freedom from biochemical failure [39].
The occurrence of hematuria following high dose rate (HDR) brachytherapy with 192-iridium has also been compared with LDR implantation using iodine-125 or palladium-103. Grills et al. compared urinary toxicity among patients treated with HDR vs. LDR modalities in 149 patients receiving treatment between 1999 and 2001 [43]. In their study with median follow of 35 months, hematuria occurred with similar frequency in both groups (p = 0.168), and did not vary by receipt of neoadjuvant androgen deprivation therapy (p = 0.164). Among 13 of 84 patients experiencing hematuria, all were grade I or II within the HDR group, while all 6 receiving 103-Pd alone experienced only grade I hematuria (Table 11.1).
Table 11.1
Radiation Therapy Oncology Group (RTOG) cooperative group common toxicity grading scale for hematuria
RTOG [34] | EORTC [44] | |
|---|---|---|
Grade I | Microscopic hematuria only | Asymptomatic; clinical or diagnostic observations only; intervention not indicated |
Grade II | Gross hematuria without clot passage | Symptomatic; urinary catheter or bladder irrigation indicated; limiting instrumental ADL |
Grade III | Gross hematuria with clot passage | Gross hematuria; transfusion, IV medications or hospitalization indicated; elective endoscopic, radiologic or operative intervention indicated; limiting self-care ADL |
Grade IV | Gross hematuria with or without clot passage requiring transfusion | Life-threatening consequences; urgent radiologic or operative intervention indicated |
Grade V | Death resulting from uncontrolled hematuria | Death |
Case 2 (This Patient Developed Severe Unremitting Hematuria following Combination Therapy)
Mr. B is a 63-year-old male with history of Gleason 4 + 3 = 7 prostate adenocarcinoma in four out of 12 cores (60 % single greatest core) and a pretreatment PSA of 11.1 ng/mL. He elected treatment with neoadjuvant androgen deprivation therapy (ADT), and palladium-103 implantation with a boost dose of external beam radiation. Following treatment, his PSA nadir is undetectable, and remained stable at 0.08 ng/mL with cessation of androgen deprivation 2 years later. He presented to the emergency room with significant pain and hematuria. A cystoscopy and clot evacuation was performed demonstrating an erythematous, friable mucosa diffusely throughout the bladder mucosa with telangiectasias noted at the bladder neck and trigone area. A biopsy was negative for malignancy but demonstrated obliterative endarteritis, fibrosis, amidst a hypovascular and hypocellular stroma. The bleeding persisted despite continuous irrigation of alum and he required numerous blood transfusions. He returned to the operating room where a cystogram was negative and was infused with 2.5 % formalin solution under general anesthesia. After a 1 month period of response, bleeding continued was refractory to a 10 % formalin installation, and a subsequent 40-treatment course of hyperbaric oxygen therapy. Ultimately he elected a cystectomy with ileal conduit for definitive therapy.
Severe hemorrhagic radiation cystitis following prostate radiotherapy is a rare and challenging complication. In contrast to the acute cellular injury mediated by the delivery of ionizing radiation leading to inflammation and edema, the clinical onset of late radiation cystitis reflects the irreversible damage to vascular and connective tissue [45, 46]. The histological characteristics include obliterative endarteritis resulting in atrophy and fibrosis which is hypovascular, hypocellular and hypoxic. It is thought that hematuria subsequently occurs as the result of necrosis of the hypoxic bladder mucosa, and may also be contributed by the rupture of superimposed dilated blood vessels (telangiectasias). Reflecting the latency of this onset, hemorrhagic cystitis has been observed between 6 months to 10 years following pelvic radiation and the severity classification of hemorrhagic cystitis exists within the RTOG and EORTC genitourinary toxicity criteria for hematuria. At presentation the performance of cystoscopy and biopsy is often required to facilitate evacuation of clot, and exclude the possibility of malignancy.
As illustrated in the preceding case, in addition to supportive care and cystoscopy with electrofulguration, the initial management typically proceeds with instillation of astringent agents to attempt blockage of the site of vascular insult. Aluminum salts (alum) may be administered intravesically following complete evacuation of organized clot at a concentration of 1 %. Aluminum toxicity (encephalopathy and cardiomyopathy) have been reported in patients receiving intravesical infusion, and therefore warrant the monitoring of serum aluminum levels, particularly in patients with renal impairment [47]. Concomitantly, control of bladder discomfort has been approached by many means including intravesical botulinum toxin administration, anticholinergics, sodium pentosan polysulfate, and conjugated estrogens [48, 49]. Formalin, a tissue fixative, has been used after failure of initial agents and following cystographic confirmation indicating the absence of vesicoureteral reflux. If reflux is demonstrated, the ureteral orifices may be first occluded with Fogarty-style catheters. Typical dilutions begin at 1 % and may be sequentially increased to a maximal concentration of 10 % [50, 51]. Irreversible fixation of the urothelium and underlying stromal and muscular architecture can lead to a significant reduction in bladder volume and contractility.
Hyperbaric oxygen is an efficacious therapy with good response rates in cases of recalcitrant radiation cystitis. In-vivo studies of irradiated tissues exposed to hyperbaric conditions demonstrated a significant increase in vascular density which may reflect a macrophage response induced at marked oxygen gradients [52]. In a series of 62 patients treated between 1988 and 2001, 86 % experienced significant improvement or complete resolution [53]. Prompt initiation of therapy following the onset of hematuria also appears to be a significant factor in response. In a study of 60 patients, 27/28 (96 %) of those treated within 6 months of onset experienced complete or partial resolution of hematuria, compared with 21/32 (66 %) treated at a longer interval (p = 0.003) [54]. While complete resolution can be achieved in many patients, the durability of these outcomes appears more modest. In a longitudinal follow-up of 11 patients only 3 had complete and sustained response, while the remaining 8 ultimately required urinary diversion as definitive therapy [55].
Following failure of initial conservative approaches, cystectomy with urinary diversion offers definitive therapy by excising the damaged urothelium. The perioperative morbidity and mortality is considerable however, with severe complications (Clavien grade III to V) occurring in 8 of 19 patients, and mortality occurring in 16 % of patients in a series of 21 patients [56]. Supravesical diversions, while sparing the added morbidity of cystectomy, leave the bladder in situ and pose a continued theoretical risk of pyocystis and secondary malignancy and are less favorable.
Case 3 (This Patients Developed a Bladder Tumor following Implant plus EBRT)
Mr. K is a 65-year-old African-American male non-smoker with a significant family history of prostate cancer, biopsy Gleason 4 + 4 prostate adenocarcinoma (six out of 12 cores, 50 % core positivity), and a pretreatment PSA of 12.7 ng/mL. He elected treatment with trimodal therapy including palladium-103 brachytherapy, neoadjuvant androgen deprivation, and combination external beam radiotherapy. His PSA nadir was 0.01 ng/mL, and is unchanged at 4 years post-implantation. He returns for evaluation following a 1-month history of intermittent gross hematuria; a voided urine cytology is positive. He undergoes cystoscopy with transurethral resection of a papillary bladder tumor. The pathology demonstrates high grade noninvasive urothelial carcinoma and he receives a 6 week course of BCG. He is recurrence-free for 2 years subsequently on surveillance cystoscopy and urinary cytology.
The incremental risk associated with brachytherapy and EBRT on the development of in-field secondary malignancies, particularly bladder cancer, is controversial. Owing to detection biases of patients receiving routine urological care, comparisons to untreated populations may not fully account for the impact of screening. Several recent publications with longitudinal follow-up appear to suggest that pelvic secondary malignancies do occur, but that the risk appears similar to that of surgically treated men. Investigators have compared cohorts of patients treated with radical prostatectomy to matched groups of men treated with intensity-modulated radiotherapy (IMRT) and brachytherapy [57]. Zelefsky and colleagues reported on 2658 men treated with either of these modalities between 1998 and 2001 at the Memorial Sloan Kettering Cancer Center. When compared to radical prostatectomy (RP), brachytherapy was not associated with an increased risk of 10-year any secondary malignancy (89 % versus 87 % p = 0.37). Among bladder cancers, 4 were detected among 413 brachytherapy patients compared with 16 of 1348 radical prostatectomy patients. In a multivariate analysis adjusting for smoking status, age, and secondary malignancy (SM) stage, brachytherapy as compared with RP was not a significant risk factor for the development of SM (p = 0.83) [32].
Moreover, the long-term experience has not suggested that bladder cancers that arise following prostate brachytherapy—with or without external beam radiotherapy—are substantively worse or exhibit higher grade features. Of 18 individuals who were subsequently diagnosed with bladder cancer from our cohort of 2532 at a median time of 3.1 years, the majority (72.2 %) of patients had low grade urothelial carcinoma, only two demonstrated muscle-invasive disease managed with cystoprostatectomy. Consequently, there is no current suggestion that patients presenting with newly diagnosed bladder cancers following brachytherapy should receive therapy that differs based on their prior radiation exposure.
Urinary Retention and Transurethral Resection of the Prostate after Brachytherapy
Case 1 (This Patient with a Large Prostate Developed Post-implant Urinary Retention)
Mr. U is a 66-year-old male with clinical T1c, Gleason 4 + 3 = 7 prostate cancer in four of 12 cores, pretreatment PSA of 5.5 ng/mL. On his diagnostic transrectal ultrasound-guided (TRUS) biopsy, his prostate volume was 64 cm 3 and his pre-implantation IPSS score was 18 with a quality of life score of 3. He elected for iodine-125 monotherapy and was placed on 6 months of neoadjuvant androgen deprivation with an LHRH agonist. At the time of implantation his prostate volume was 40 cm 3 . The procedure was uneventful and the post-procedure cystogram normal. He voided spontaneously in the recovery room prior to discharge but returned to the emergency room with urinary retention 24 h later. A Foley catheter was placed and he was started on an oral alpha-blocker. After 1 week of catheter drainage, he voided spontaneously and had a post-void residual of 10 mL.
Acute urinary retention (AUR) is a common event following prostate brachytherapy, occurring in as many as 34 % of patients in some series, though the onset and duration may vary [58–61]. The etiology of AUR is generally attributed to obstructive edema of the prostatic urethra, where prostatic volume may increase by as much as 18 % post-implantation [62]. Many clinical risk factors appear to contribute to this outcome including diabetes, larger prostate size, transition zone volume and higher urinary symptom score [63]. Practitioner experience also appears to affect retention rates, possibly reflecting a learning curve in minimizing urethral inflammation [64]. Following catheter placement, the majority of patients experiencing retention will spontaneously void after a short interval of drainage. The addition of alpha blocker therapy, particularly in men with high baseline urinary symptom scores, seems warranted.
The administration of neoadjuvant androgen deprivation in this patient reflects the oncologic benefit born out of randomized evidence in support of a synergistic effect with radiation [65, 66]. In addition to patients with intermediate and high risk disease, we have opted for a risk adapted approach to neoadjuvant ADT. Based on our findings that ADT confers a fourfold risk reduction of retention in patients with prostate volume greater than 50 cm3 and IPSS >15, it has become our practice to give ADT to these patients [7]. Investigators have also attempted to target the inflammatory response that may underlie post-brachytherapy retention with peri-procedural meloxicam in a randomized trial of 300 patients; however no differences were observed [67].
Patients with persistent urinary retention may be managed with extended catheter drainage, suprapubic tube placement, or clean intermittent catheterization while awaiting resolution of inflammation. The impact on health related quality of life (HRQoL) associated with extended periods of catheter drainage is not trivial. In one study of 127 patients undergoing low dose rate iodine-125 implantation in which AUR occurred in 13 patients, quality of life scores in these patients were lower at study endpoints [68]. Three patients were managed with suprapubic tube drainage and nine ultimately received transurethral resection of the prostate. For men with retention refractory to catheter drainage and medical therapy, transurethral resection of the prostate may be warranted but is associated with a considerable risk of urinary incontinence [69–71].
Case 2 (This Patient Developed Post-implant Retention and Required a TURP)
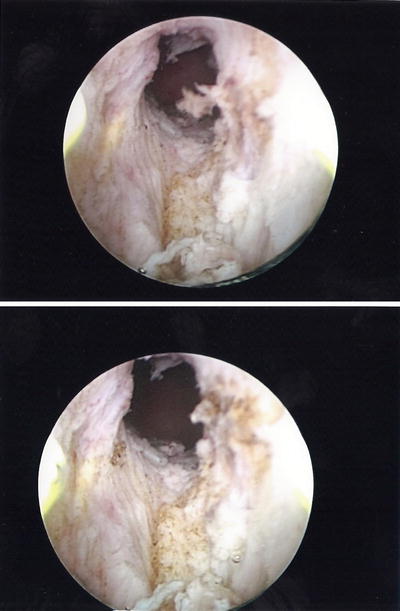
Mr. G is a 70-year-old male with history of Gleason 3 + 3 prostate cancer in six out of 12 biopsy cores (50 % in a single highest core), pretreatment PSA 6.1 ng/mL. On TRUS biopsy his prostate volume was 45 cm 3 and a multiparametric prostate MRI demonstrated no evidence of T2 signal abnormality, extra-prostatic extension or restricted diffusion however an intravesical median prostatic lobe was noted. His baseline IPSS score was 8. He elected treatment with brachytherapy monotherapy (iodine-125). His procedure was uneventful, however he was unable to void after catheter removal. No improvement was seen after a trial of alpha-blockers, and a suprapubic tube was placed after 6 weeks of catheter drainage. A urologist performed a limited transurethral resection of the prostate (TURP) (Fig. 11.3 ) and the patient did well after the procedure.