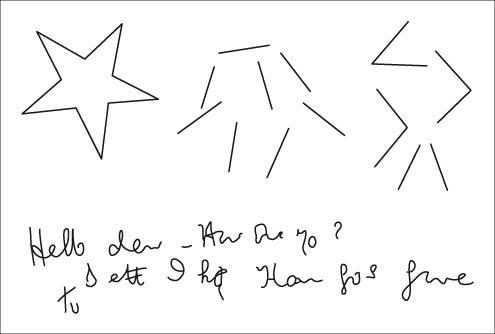
Below: writing difficulties are common. The patient is attempting to write: ‘Hello dear. How are you? Better I hope. That goes for me too’.
Early signs of disturbed consciousness include a reduction of spontaneous movement, a fixed stare, apathy, and slowness and brevity of responses. Day-time sleepiness may be a feature but the disturbances in night-time sleep behaviour, commonly observed in this population, are not, as generally believed, related to the presence of hepatic encephalopathy [3]. Coma at first resembles normal sleep, but further deterioration results in reaction only to intense or noxious stimuli progressing to complete unresponsiveness. Rapid changes in the level of consciousness are accompanied by delirium.
Foetor hepaticus, a sour, musty, faeculent smell, can be detected on the breath of some patients; it is attributed to the presence of mercaptans [4]. Its presence does not correlate with the degree or duration of encephalopathy and its absence does not exclude this condition.
The changes in motor function include rigidity, disorders of speech production, resting- and movement-induced tremor, asterixis, delayed diadochokinetic movements, hyper- or hyporeflexia, choreoathetoid movements, Babinsky’s sign and transient focal symptoms [1,5–8]. Severe motor abnormalities are easily detected but subtle motor abnormalities, particularly mild extrapyramidal symptoms such as bradykinesia, rigidity and resting tremor, are less easily recognized [8].
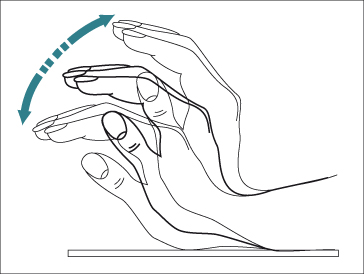
Asterixis (flapping tremor) is the best known motor abnormality. It is caused by impaired inflow of joint and other afferent information to the brainstem reticular formation, resulting in arrhythmic lapses in posture. The tremor is absent at rest, less marked on movement and maximum on sustained posture. It is best demonstrated by asking the patient to stretch out their arms and hyperextend the wrists with separated digits and their forearm fixed (Fig. 8.2). The rapid flexion–extension movements at the metacarpophalangeal and wrist joints are often accompanied by lateral movements of the digits. The tremor is usually bilateral, although not bilaterally synchronous with one side affected more than the other. It may also be appreciated by asking the patient to tightly grip the examiner’s hand. Similar changes may be observed in the arms, neck, jaw, protruded tongue, and tightly closed eyelids and the gait may be ataxic. A ‘flapping’ tremor is not specific for hepatic encephalopathy; it can also be observed in renal failure, respiratory failure, severe heart failure, hypomagnesaemia, and diphenylhydantoin intoxication.
Speech is slow and slurred and the voice is monotonous. In deep stupor, dysphasia becomes marked and is always combined with perseveration.
Deep tendon reflexes are usually exaggerated. Increased muscle tone is present at some stage and sustained ankle clonus is often associated with rigidity. During coma, patients become flaccid and lose their reflexes. The plantar responses are usually flexor becoming extensor in the deepest stages of coma.
Excessive appetite, muscle twitching, grasping and sucking reflexes may also be seen. Visual disturbances, including reversible cortical blindness [9] and alternating gaze deviation [10], have been reported.
These individuals also show a wide spectrum of other abnormalities, including impaired psychomotor performance [11,12], disturbed neurophysiological function [13–17], altered cerebral neurochemical/ neurotransmitter homeostasis [18,19], reductions in global and regional cerebral blood flow and metabolism [20,21], and changes in cerebral fluid homeostasis [22].
The abnormalities observed in psychometric, neurophysiological and neural imaging studies, in patients with overt hepatic encephalopathy, do not necessarily correlate with one another or with the degree of impairment observed clinically, although, in general, the abnormalites increase as the clinical condition worsens.
Episodic Hepatic Encephalopathy
Episodic hepatic encephalopathy arises in patients who have previously been clinically stable. In approximately 50% of such individuals an obvious precipitant can be identified (Table 8.1). These precipitating factors compromise the patient by: (i) further depressing hepatic or cerebral function; (ii) increasing the nitrogenous load; or (iii) stimulating an inflammatory response. Hepatic function may be depressed by fluid loss and a reduction in hepatic perfusion following gastrointestinal bleeding, diuretic over-usage, large paracenteses and diarrhoea/ vomiting, or during an episode of sepsis. Gastrointestinal bleeding will also substantially increase the intestinal nitrogen load as will dietary protein excess and constipation. Cerebral function may be compromised by infection and inflammation and following ingestion of alcohol, which is poorly tolerated. Excessive sensitivity to the cerebral effects of a number of psychoactive drugs, especially opiates and benzodiazepines, is often observed [23]. Surgical procedures are tolerated extremely poorly. The creation of surgical or transjugular intrahepatic portal–systemic shunts (TIPS) may precipitate or worsen hepatic encephalopathy by increasing the amount of toxin-laden blood that impinges on the brain.
Table 8.1. Precipitants of hepatic encephalopathy in patients with cirrhosis
| Gastrointestinal bleeding |
| Sepsis |
| Electrolyte imbalance |
| Hyponatraemia |
| Hypokalaemia |
| Dehydration |
| Fluid restriction |
| Excessive diuresis |
| Paracentesis |
| Diarrhoea/ vomiting |
| Constipation |
| Excess protein load |
| Alcohol misuse |
| CNS-active drugs |
| TIPS insertion |
| Surgery |
TIPS, transjugular intrahepatic portal–systemic shunt.
Patients may return to normal following an episode of overt hepatic encephalopathy; the improvement in their clinical state is usually apparent before improvements in psychometric tests or the electroencephalogram (EEG). However, many will retain some degree of clinical, neuropsychometric or neurophysiological impairment in the longer term [24], particularly those with severely decompensated liver disease or those with large spontaneous or surgically created portal-systemic shunts.
Persistent Hepatic Encephalopathy
A small number of patients show persistent but stable evidence of hepatic encephalopathy. Many of these have extensive portal–systemic shunting, either multiple anastomotic channels or, more often, one major collateral shunt. In some the shunt may be surgically created or inserted as a TIPS. Some fluctuation in the clinical picture may be observed in relation to various precipitant, which usually manifests as a worsening of the predominant clinical features rather than by changes in conscious level. Parkinsonian features may be prominent with a fine tremor unaffected by intention, pronounced rigidity, staccato speech and a shuffling gait. Cerebellar features are often encountered, manifesting as gait disturbance, truncal ataxia, an intention tremor and dysarthria. Involuntary choreoathetoid movements may be observed. Clinical and biochemical evidence of liver disease may be equivocal or absent, and the neuropsychiatric disorder may dominate the picture. In consequence, the diagnosis is often missed.
Hepatic Myelopathy
Hepatic myelopathy develops predominantly in men who have undergone surgical portal–systemic shunting and is far less common, and less well-defined, than hepatic encephalopathy. It presents as a progressive, spastic paraparesis without sensory impairment or sphincter dysfunction [25,26]; the clinical syndrome is accompanied by degenerative changes in the spinal cord.
Minimal Hepatic Encephalopathy
The term minimal hepatic encephalopathy is used to describe patients with cirrhosis who are ‘clinically normal’ but who show abnormalities of cognition and/or neurophysiological variables [12,27]. Use of this term has the advantage that it allows hepatic encephalopathy to be considered as a single syndrome with quantitatively distinct features relating to severity but it also has the disadvantage that it might not adequately convey the fact that its presence is not without consequence. It has a detrimental effect on health-related quality of life [28,29] and the ability to perform complex tasks, such as driving [30,31]; it also increases the risk of developing overt hepatic encephalopathy [16,32–34].
Diagnosis
The diagnosis may be easy—for example in a patient with known cirrhosis and gastrointestinal haemorrhage or sepsis, who, on admission, is confused and has a ‘flapping’ tremor. However, without the clinical background data, and an obvious precipitating event, hepatic encephalopathy may go unrecognized, and thus untreated.
There is no gold standard for diagnosing hepatic encephalopathy in patients with cirrhosis. There are a number of individual techniques that access different aspects of cerebral function, which can be used, singly or in combination, to provide diagnostic information. These include mental state assessment, psychometric testing, electroencephalography, sensory and cognitive evoked potentials, and neuroimaging. In practice, any measure that has a proven relationship with the behavioural, prognostic and, possibly, pathophysiological features of this syndrome can be used as a surrogate marker.
Neurological Examination Including Mental State Assessment [35]
In patients with cirrhosis, the diagnosis or exclusion of overt, or clinically apparent, hepatic encephalopathy should be based on:
1 a careful and detailed neuropsychiatric history and examination, with particular attention paid to changes in mental state, for example memory, concentration, cognition and consciousness, and to changes in energy and activity levels, and overall health-related quality of life;
2 use of two-grading systems to assess mental status: the West Haven criteria [36] (Table 8.2), based on changes in consciousness, intellectual function and behaviour, and the Glasgow Coma Scale [39] (Table 8.3). Additional instruments such as the Hodkinson Mental State Test [40] or the Mini Mental Score Test [41], which have been widely applied in this patient population, can also be used;
3 a comprehensive neurological examination looking particularly for evidence of subtle motor abnormalities, including: hypomimia, dysarthria, increased tone, reduced speed or difficulty executing rapid, alternating movements, ataxia, increased deep tendon reflexes, impaired postural reflexes and abnormal movements such as tremors, particularly asterixis; the presence of sensory change and/or focal features would suggest an alternative or additional diagnosis;
4 the exclusion of other potential causes of neuropsychiatric abnormalities, for example concomitant neurological disorders such as subdural haematoma and Wernicke’s encephalopathy, other metabolic abnormalities such as those associated with diabetes and renal failure, and intoxication with alcohol or drugs.
Table 8.2. West Haven Criteria for grading mental state in patients with cirrhosis*
| Grade | Features |
| 0 | No abnormalities detected |
| I | Trivial lack of awareness |
| Euphoria or anxiety | |
| Shortened attention span | |
| Impairment of addition or subtraction | |
| II | Lethargy or apathy |
| Disorientation for time | |
| Obvious personality change | |
| Inappropriate behaviour | |
| III | Somnolence to semi-stupor |
| Responsive to stimuli | |
| Confused | |
| Gross disorientation | |
| Bizarre behaviour | |
| IV | Coma, unable to test mental state |
* The descriptions of the mental state alterations in hepatic encephalopathy are those originally proposed by Conn et al. [36] as a modification of Parsons-Smith criteria [13]. Alternative formulations have been proposed by Blei and Córdoba [37], Ferenci et al. [2] and Amodio et al. [38].
Table 8.3. The Glasgow Coma Score [39]
| Variable | Score |
| Eye open | |
| Spontaneously | 4 |
| To command | 3 |
| To pain | 2 |
| No response | 1 |
| Best motor response | |
| Obeys verbal commands | 6 |
| Painful stimulus, localizes pain | 5 |
| Painful stimulus, flexion/ withdrawal response | 4 |
| Painful stimulus, abnormal flexion | 3 |
| Painful stimulus, extension | 2 |
| No response | 1 |
| Best verbal response | |
| Orientated and conversant | 5 |
| Disorientated and conversant | 4 |
| Inappropriate words | 3 |
| Incomprehensible sounds | 2 |
| No response | 1 |
| Total score | 3 (Worst) to 15 (Best) |
It would clearly be sensible to obtain corroborative reports from relatives or friends, particularly in relation to observed rather than subjective changes in behaviour and mental state.
Psychometric Performance [35,42]
Impaired psychometric performance defines the presence of minimal hepatic encephalopathy in patients with cirrhosis who appear clinically unimpaired. It also provides an objective measure of severity in those with obvious clinical impairment. Patients with minimal hepatic encephalopathy show deficits in attention, visuospatial abilities, fine motor skills and working memory while other cognitive abilities are relatively preserved. Patients with overt hepatic encephalopathy show additional disturbances in psychomotor speed, executive function and concentration [43,44].
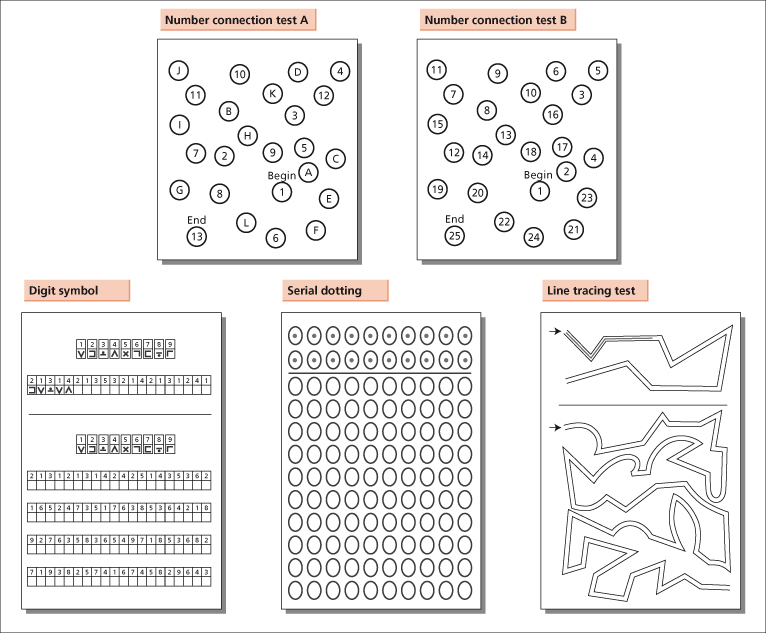
Attempts have been made to develop a simple but comprehensive diagnostic test battery based on the deficits in psychomotor function identified in this patient population. Test batteries are generally more reliable than single tests, and tend to be more strongly correlated with functional status. Promising results have been obtained using five paper and pencil tests, namely Number Connection Tests A and B, and the line tracing, serial dotting and digit symbol tests [12,44] (Fig. 8.3). This battery, which has been called the Psychometric Hepatic Encephalopathy Score (PHES), assesses the required domains of attention, visual perception and visuoconstructive abilities; it is easily applied and has been shown to have a high specificity for the diagnosis of hepatic encephalopathy. However, test scores have to be normalized for a number of confounding variables and at present normative databases are only available for German, Italian, Spanish and British populations.
Fig. 8.3. The five paper and pencil tests that make up the Psychometric Hepatic Encephalopathy Score (PHES), which assesses attention, visual perception and visuoconstructive abilities [12,44]. Number Connection Tests A and B: subjects are asked to join the numbers or numbers and letters in sequence as quickly as possible. The time taken to complete the task is recorded. Digit Symbol Test: subjects are asked to insert symbols in the blank squares below the numbers using the key provided. The exercise is timed and the number correctly completed in 90 seconds recorded. Serial Dotting: subjects are asked to place a dot in the centre of each circle and to complete the page as quickly as possible. The time taken to complete the task is recorded. Line Tracing: subjects are asked to trace a line between the two guidelines as quickly and accurately as possible without moving the paper. The time taken to complete the task and the number of errors made are recorded.
Computer-based psychometric tests may allow a more precise quantification of reaction times and more refined testing. Instruments such as the Scan test, based on the Sternberg paradigm, tend to explore better-defined cognitive functions and to isolate subtle attention or memory defects [45].
Neurophysiology [35,46]
Electroencephalography (EEG)
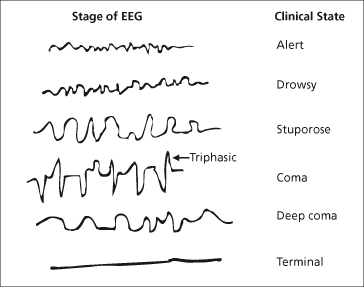
The EEG primarily reflects cortical neuronal activity. Hepatic encephalopathy is characterized by a progressive slowing of the normal alpha frequency of 8 to13 Hz. Bursts of slow activity are observed in the theta (4–8 Hz) range, initially in the temporal areas, and then more diffusely over the scalp; further slowing into the delta (1–4 Hz) range may then occur. Triphasic waves or arrhythmic delta activity occur with more severe grades of encephalopathy; coma is characterized by slow, low-voltage delta activity with sequences of electric silence (Fig. 8.4).
Fig. 8.4. EEG changes in patients with cirrhosis with increasing deterioration in neuropsychiatric status. There is an initial slowing in frequency with increasing amplitude. The amplitude then decreases. Finally there is an absence of rhythmic activity.
The generalized slowing of the background EEG activity and the development of triphasic waves is not specific for hepatic encephalopathy; these changes are also observed in other metabolic encephalopathies (uraemia, hypocapnia and hyponatraemia) and in drug-induced encephalopathies (lithium, valproate and baclofen) [47]. However, these conditions are usually easily distinguished on clinical grounds.
The sensitivity of the EEG for the diagnosis of hepatic encephalopathy varies. The best results are probably obtained using spectral analysis-based techniques [48]. Abnormalities of the EEG are reported in 43 to 100% of patients with overt hepatic encephalopathy and in 8 to 40% of clinically unimpaired patients with cirrhosis [35,49].
Recent advances in EEG analysis, such as fully automatic evaluation based on artificial neural networks [50], and techniques for providing spatial as well as temporal information [17], should provide better quantifiable and more informative data.
Evoked Potentials
Sensory or exogenous evoked potentials (EPs) are generated by the passive reception of sensory stimuli triggered by visual, auditory or peripheral nerve (somatosensory) stimulation. They provide information on both cortical and brainstem activity. Abnormalities of exogenous EPs which reflect cortical function have been described in patients with both minimal and overt hepatic encephalopathy. However, the data available are inconsistent; methodologies and data interpretation need to be standardized before recommendations can be made.
Cognitive or endogenous EPs are triggered by cognitive activity. The best known is P300 which is triggered when the subject receives an infrequent visual or auditory stimulus embedded in a series of otherwise irrelevant, frequent stimuli. The potential occurs about 300 ms after exposure to the rare stimulus, hence its name. Assessment of P300 latency has diagnostic potential for detecting the presence of minimal hepatic encephalopathy and for monitoring the status of patients with mild to moderate hepatic encephalopathy over time [34]. The techniques currently employed are not suitable for use in patients with higher grades of encephalopathy because of the need for patient cooperation.
Critical Flicker Fusion Frequency
Critical flicker fusion frequency (CFF) is a technique that centres on the perception of light as flickering or fused as its frequency changes. In one study a threshold of 39 Hz in the flicker frequency completely separated patients with overt hepatic encephalopathy from their unimpaired counterparts; it separated cirrhotic patients with minimal hepatic encephalopathy from unimpaired individuals with a sensitivity of 55% and a specificity of 100% [51]. The utility of CFF for the diagnosis and monitoring of hepatic encephalopathy has been confirmed by others [52–55]. However, the technique requires patient cooperation and is not sufficiently sensitive enough to be used as a stand alone technique for the detection of minimal hepatic encephalopathy.
Smooth Pursuit Eye Movements
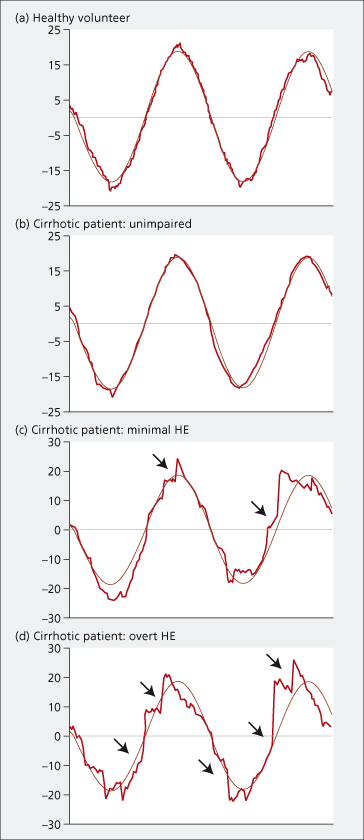
Smooth pursuit eye movements (SPEM) are the conjugate movements used to track, or pursue, the smooth trajectory of small targets. SPEM recordings show clear disruption of smooth pursuit in patients with minimal hepatic encephalopathy, and more pronounced disruption, if not complete loss of smooth pursuit, in patients with overt hepatic encephalopathy [56] (Fig. 8.5). The abnormalities in pursuit behaviour mirror the changes observed in clinical status and psychometric performance over time and following treatment. The technique requires patient cooperation and can not, therefore, be used across the whole spectrum of neuropsychiatric impairment. It does, however, provide insights into motor function which are not otherwise easy to access.
Fig. 8.5. Smooth pursuit movements in a healthy volunteer and in three individual patients with cirrhosis, by degree of neuropsychiatric impairment. The dot position is denoted in light gray, and the eye position in red. In the healthy volunteer (a) and in the unimpaired cirrhotic patient (b), pursuit is smooth. In the patient with minimal hepatic encephalopathy (c), pursuit is smooth but interspersed with corrective catch-up saccades (arrowed). In the patient with overt hepatic encephalopathy (d), pursuit is no longer smooth but accomplished by a series of corrective catch-up saccades (arrowed) producing a jerky or cogwheel pattern. HE: hepatic encephalopathy
(Adapted from [56], with permission).
Functional and Structural Cerebral Imaging [57,58]
The development of computed X-ray tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), single photon emission tomography (SPET) and positron emission tomography (PET), and their functional imaging counterparts, has enabled rapid and non-invasive assessments of cerebral structure and metabolism to be made.
Cerebral CT and MR Imaging [59,60]
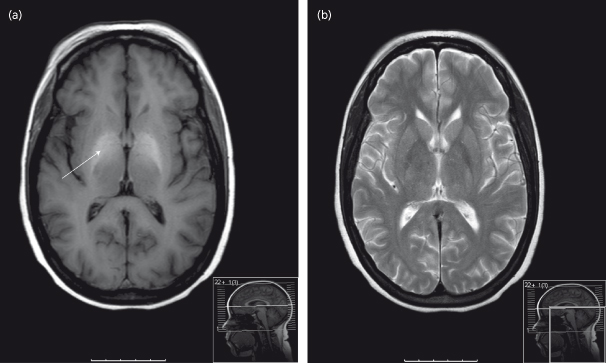
Conventional cerebral CT and MR imaging may show evidence of cerebral and sometimes cerebellar atrophy in patients with cirrhosis. These changes are related to the severity of the liver dysfunction and do not relate to changes in cerebral function. They are most prominent in patients with a history of alcohol abuse. Hyperintensity in the basal ganglia on MRI T1-weighted images may also be observed but does not correlate with either the presence or severity of hepatic encephalopathy (Fig. 8.6). These changes probably reflect pallidal deposition of manganese. Total body manganese is increased in patients with cirrhosis most likely reflecting the combined effects of hepatocellular failure, impaired biliary excretion and the presence of portal–systemic shunting of blood [61–65]. It is not clear whether the manganese deposits in the globus pallidus reflect intoxication or the presence of an adaptive process designed to improve the efficacy of ammonia detoxification by astrocytes. However, manganese accumulation may be responsible, at least in part, for up-regulation in the cerebral peripheral benzodiazepine receptors which play a role in the pathogenesis of hepatic encephalopathy (see below) [66,67].
Fig. 8.6. T1– and T2-weighted MR images of the brain of a 53-year-old individual with cirrhosis and overt hepatic encephalopathy. (a) The T1-weighted MR image shows bilateral, symmetrical hyperintensity of the globus pallidus (arrowed). (b) No corresponding changes are observed in the T2-weighted MR image.
Cerebral imaging, using these modalities, does not provide information of diagnostic importance in patients with cirrhosis and hepatic encephalopathy except to exclude other causes of cerebral dysfunction. However, newer MRI techniques may prove more informative. Magnetization transfer and diffusion-weighted imaging allow indirect assessment of changes in cerebral water content and distribution. Volumetric MRI provides precise measurements of whole or regional brain volumes and allows small difference in brain size to be monitored (co-registered MRI). Functional MRI provides measurements of the haemodynamic responses related to neural activity in the brain.
Cerebral MRS [68]
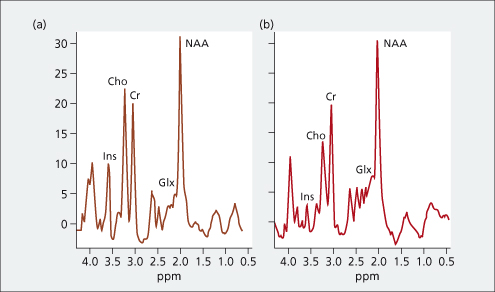
In vivo MRS can provide localized biochemical information on cerebral metabolic processes. Characteristic alterations have been observed in cerebral 1H MRS in patients with cirrhosis, which correlate with the degree of neuropsychiatric impairment. These alterations, which are typified by relative reductions in the myoinositol and choline resonances and a relative increase in the composite glutamine/ glutamate resonance, are thought to reflect changes in astrocyte volume homeostasis (Fig. 8.7). Possible advances in the field, including two-dimensional spectroscopy, spectral editing and the use of higher magnetic field strengths, may help resolve some of the current technical difficulties.
Fig. 8.7. 1H-MR-spectroscopy water-suppressed spectra in a healthy individual (a) and in a patient with cirrhosis and hepatic encephalopathy (b) recorded with a stimulated echo acquisition mode pulse sequence (TR/TE, 1600/20 ms; acquisitions, 256). The main resonances correspond to N-acetylaspartate (NAA: 2.0 ppm), glutamine/ glutamate (Glx, 2.1–2.5 ppm), creatine/ phosphocreatine (Cr: 3.02 ppm), choline-containing compounds (Cho: 3.2 ppm) and myoinositol (Ins: 3.55 ppm). The presence of hepatic encephalopathy is characterized by a relative increase in the glutamate/ glutamine resonance and relative reductions in the myoinositol and choline resonances.
Radiotracer Imaging [58]
Radiotracer imaging with SPECT and PET allows access to metabolic processes, neuronal activity and neurotransmitter systems. However, these are expensive and generally inaccessible techniques. They provide considerable insights into the pathophysiology of hepatic encephalopathy but currently have no place in the management of patients.
Blood Ammonia
Measurement of blood ammonia may be of value in the differential diagnosis of hepatic encephalopathy. It can be particularly useful when a patient not known to have cirrhosis presents with fluctuating neurological symptoms and signs of seemingly unknown origin. The signs of chronic liver disease are likely to be minimal and the liver function tests are unlikely to be severely disturbed. In this instance measurement of blood ammonia may provide a vital clue. The pH-dependent partial pressure of gaseous ammonia in arterial blood correlates more closely with the clinical and neurophysiological changes observed than plasma ammonia concentrations [69].
Cerebrospinal Fluid
The cerebrospinal fluid (CSF) is usually clear and under normal pressure. Patients with severe Grade III/IV encephalopathy may have increased CSF protein concentrations, but the cell counts are normal. CSF glutamine concentrations may be increased and correlate significantly with both the presence and the degree of hepatic encephalopathy [70].
Neuropathological Changes [71,72]
Examination of brain tissue is rarely, if ever, undertaken during life. Microscopically the most striking feature is proliferation of the astrocytes with development of enlarged nuclei, prominent nucleoli, margination of chromatin and accumulation of glycogen—changes referred to as Alzheimer type II astrocytosis. These changes are found particularly in the cerebral cortex, basal ganglia and cerebellum. Microglial changes may also be observed; neurones show only minor, if any, alterations.
More significant changes are seen in patients with persistent hepatic encephalopathy including: patchy cortical laminar or pseudolaminar necrosis with polymicrocavitation at the corticomedullary junctions and in the striatum and uneven degeneration of neurones and medullated fibres in the cerebral cortex, cerebellum and lenticular nuclei.
Demyelination in the pyramidal tracts is observed in patients with hepatic myelopathy.
Choice of Diagnostic Variables [58]
There are a number of techniques which provide information useful for the diagnosis and monitoring of hepatic encephalopathy in patients with cirrhosis. Ideally, the methods used should: (1) access variables germane to the pathophysiology of the syndrome; (2) have predictive validity; and (3) not expose the patient to unnecessary risk or harm. In practice, the selection of diagnostic tools will be determined by factors such as simplicity of use, accessibility and cost.
Current guidelines suggest: (1) a detailed clinical assessment to identify or exclude clinically apparent change; (2) an assessment of psychometric performance using the PHES battery or a validated computer-based system; (3) an electrophysiological assessment, for example an EEG or somatosensory/ cognitive EPs, if accessible; and (4) an assessment of health-related quality of life [2]. It is unlikely that these guidelines are followed, except perhaps in centres with on-going research interests. Most patients are probably suboptimally assessed by clinical examination alone or, at best, with the addition of a few simple psychometric tests. This is not a satisfactory situation but one which may improve if simpler tools, such as CFF, can be further validated.
Differential Diagnosis
Neuropsychiatric abnormalities may arise in patients with cirrhosis that do not relate to the presence of hepatic encephalopathy. The clinician should be alert to the possibility of intracranial haemorrhage, cerebral trauma, infection or tumour, as well as possible drug-induced or other metabolic encephalopathies. Particular difficulties arise when faced with the occurrence of one or more confounding or competing events. The diagnosis is even more difficult to make if the patient is not known to have cirrhosis; then the origin of the neuropsychiatric abnormalities can not be appreciated and may prove elusive to the detriment of the patient.
Hyponatraemia, defined as a serum sodium of less than 136 mmol/L, can result in cerebral over-hydration and a metabolic encephalopathy [73]. Nausea and malaise are the earliest findings. Headache, lethargy and eventually seizures, coma and respiratory arrest will follow if the plasma sodium concentration continues to fall. Hyponatraemia is frequent among cirrhotic patients primarily as a result of fluid retention, although dietary sodium restriction, diuretic over-usage and paracentesis may play a role. Patients with cirrhosis may, therefore, manifest features of both hyponatraemic and hepatic encephalopathy [73]. Moreover, hyponatraemia may precipitate or worsen existing hepatic encephalopathy [74]. Hyponatraemia is a risk factor for the subsequent development of overt hepatic encephalopathy in patients undergoing TIPS [75].
The features of alcohol withdrawal may confound the clinical picture. Delirium tremens is distinguished by continuous motor and autonomic over-activity, profound insomnia, terrifying hallucinations and a finer, more rapid tremor. The patient is flushed, agitated, inattentive and perfunctory in their replies. Tremor, absent at rest, becomes coarse and irregular on activity. Treatment of alcohol withdrawal in a patient with cirrhosis is difficult and patients must be closely monitored. The sedation required may precipitate hepatic encephalopathy so it is best to start prophylactic antiencephalopathy treatment from the outset.
Wernicke’s encephalopathy is caused by thiamine deficiency and is most commonly seen in patient with a long history of alcohol misuse and a degree of malnutrition. It presents as an acute neuropsychiatric condition characterized by global confusion, eye signs and ataxia. The confusional state is accompanied by apathy, disorientation and disturbed memory, but drowsiness and stupor are uncommon. The ocular abnormalities include nystagmus, gaze palsies and ophthalmoplegia, while the ataxia affects the trunk and lower extremities. The clinical abnormalities may develop acutely or evolve over several days. Particular diagnostic difficulties arise in patient with alcoholic cirrhosis who are actively withdrawing from alcohol and who may in addition develop hepatic encephalopathy. Prophylactic parenteral thiamine should be given over several days to patients in this situation.
Wilson’s disease, a condition of disordered copper metabolism, can cause both cirrhosis and neuropsychiatric abnormalities. Patients tend to present in early life but some may present later. Initially, they may exhibit mild cognitive deterioration and clumsiness, as well as changes in behaviour. Specific neurological symptoms then follow, including parkinsonism with or without a typical hand tremor, masked facial expressions, slurred speech, ataxia or dystonia. These neurological features may be accompanied by depression, anxiety and psychosis. However, the symptoms do not fluctuate; Kayser–Fleischer rings and disturbances in copper metabolism can usually be demonstrated and serve to differentiate.
Latent functional psychoses, such as bipolar disorder, may be precipitated by the onset of hepatic encephalopathy. Conversely, major psychoses may develop in patients with chronic liver disease independently of the presence of hepatic encephalopathy. The diagnosis is difficult; a previous history of mental health disorder and the response to anti-encephalopathy treatment may help clarify. The medical management of these patients can be difficult, particularly if major anti-psychotic medication is required.
Hepatic Encephalopathy and Liver Transplantation
Significant difficulties arise in assessing patients for liver transplant when the only indication appears to be cognitive decline or the relatively fixed neurological abnormalities characteristic of persistent hepatic encephalopathy. In these instances attempts must be made to distinguish these changes from other causes of neurocognitive/ neurological impairment.
It might be imagined that abnormalities on cerebral imaging would favour an alternative pathology but this can not be relied upon with certainty. Mild to moderate brain atrophy is found in the majority of patients with long-standing hepatic encephalopathy, particularly those with alcohol-related cirrhosis, and does not necessarily indicate progressive dementia. Brain size may further decreases after liver transplantation due to the resolution of low-grade brain oedema but improvements are still seen in cognitive function [76].
Focal white matter lesions are a feature of several types of small-vessel cerebrovascular disease and are strongly associated with advanced age, high blood pressure, diabetes, stroke and myocardial infarction. They remain permanently visible on cerebral MR images and do not resolve. Their presence is associated with cognitive impairment, depression and gait abnormalities. Focal white matter lesions, radiologically indistinguishable from those associated with small vessel disease, are seen in the brains of patients with cirrhosis. However, they can markedly decrease in volume after liver transplantation. Thus, their presence, even if extensive, does not necessarily indicate that the patient’s cognitive impairment is of vascular origin [76,77]. These lesions most probably reflect changes associated with the presence of low-grade brain oedema.
Advances in functional MR imaging and more widespread application of MRS may help resolve some of these issues. Currently, the best approach is to assess the degree of reversibility of the neuropsychiatric symptoms by maximizing treatment, if necessary in hospital so that compliance can be assured. Objective measures should be used to monitor improvement, preferably a combination of neuropsychometric and neurophysiological variables.
Prognosis
The presence of hepatic encephalopathy is associated with significant impairment in the ability to perform complex tasks, such as driving [30,31], a detrimental effect on health-related quality of life [28,29] and a substantial financial burden on health-care systems [78]. Its presence, in patients with cirrhosis, is also associated with a significant reduction in survival. Thus, the survival probability, after a first episode of an acute hepatic encephalopathy, is 42% at 1 year and 23% at 3 years [79].
Pathogenesis
The pathogenesis of hepatic encephalopathy has caused more controversy and division over the years than almost any other topic in hepatology. Many hypotheses have been proposed; most have been hotly contested and several, for example the false neurotransmitter theory and the gut–derived γ-aminobutyric acid (GABA) theory, have been abandoned. Any hypothesis regarding the pathogenesis of this syndrome must adequately explain the following:
1 the broad spectrum of clinical findings which appear to reflect dysfunction of multiple cerebral systems;
2 the fluctuant nature of the clinical picture, in particular its rapid evolution and its complete reversibility;
3 the mechanisms by which a variety of diverse condition precipitate deterioration in the clinical picture;
4 the mechanisms that result in alleviation of the clinical symptoms in response to treatment.
Recent advances in cellular and molecular biology, and in human non-invasive brain imaging/ quantification, have resulted in considerable progress in understanding the pathogenesis of this syndrome. In consequence, although there are still uncertainties, the emerging picture allows a number of individual findings, none of which explain the syndrome in its entirety, to be subtly integrated into a synergistic whole.
Key Concepts and Contributors
The two key players in the development of hepatic encephalopathy, in patients with cirrhosis, are hepatocellular failure and portal–systemic shunting. Portal–systemic shunting, in the absence of liver disease, for example following portal vein thrombosis, is not usually accompanied by the development of significant hepatic encephalopathy. However, creation of a surgical shunt or TIPS insertion in patients with chronic liver disease can precipitate or worsen existing neuropsychiatric change. Likewise, the presence of extensive portal–systemic shunting in the presence of well-preserved liver function, as in patients with schistosomal liver injury, is not usually associated with the development of major neuropsychiatric impairment.
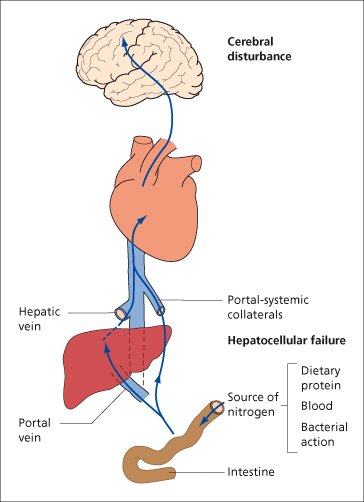
In the presence of hepatocellular failure and portal–systemic shunting, the hepatic clearance of gut-derived neurotoxic material is impaired. This material impinges on the brain, resulting in both direct and indirect impairment of astrocyte function (Fig. 8.8). Complex changes then follow which involve brain water homeostasis, oxidative and nitrosative stress, cerebral neurotransmitters and possibly inflammation; the net effect is disruption of glioneuronal communication and neuronal function.
Fig. 8.8. Hepatocellular failure and portal–systemic shunting are key players in the development of hepatic encephalopathy in patients with cirrhosis. In the presence of these complications the hepatic clearance of gut-derived neurotoxic material is impaired. The neurotoxic material impinges on the brain, resulting in both direct and indirect impairment of astrocyte function. Complex changes then follow, which ultimately disrupt glioneuronal communication and neuronal function.
Thus, there are a number of key factors that determine the development of hepatic encephalopathy (Table 8.4). These will be discussed individually. An attempt will then be made to integrate them into a cohesive model.
Table 8.4. Key factors in the pathogenesis of hepatic encephalopathy
| Gut-derived neurotoxins |
| Brain water homeostasis |
| Oxidative/ nitrosative stress |
| Astrocyte dysfunction |
| Neurotransmitter dysfunction |
| Infection and inflammation |
Gut-Derived Neurotoxins
Several gut-derived neurotoxins have been implicated in the pathogenesis of hepatic encephalopathy; by far the most important is ammonia.
Ammonia is produced in the intestine from dietary protein, deamination of glutamine via glutaminase and bacterial action in the colon. It is absorbed by non–ionic diffusion; concentrations in the portal vein are tenfold higher than in arterial blood. The hepatic extraction rate is high. The ammonia in portal blood, together with the ammonia derived from hepatic amino acid metabolism, is taken up by periportal hepatocytes and metabolized to urea via the urea cycle. Some ammonia is taken up by perivenous hepatocytes where it is converted to glutamine via glutamine synthetase. These two systems, working in concert, tightly control blood ammonia concentrations in the hepatic veins.
Blood ammonia levels may be increased in patients with cirrhosis for a number of reasons: (1) small bowel colonization with urease-containing bacteria; (2) enhanced intestinal absorption of ammonia secondary to the increased splanchnic blood flow associated with portal hypertension; (3) intra- and extra-hepatic portal systemic shunting; (4) reduction in functioning hepatocyte mass; (5) decreased ammonia metabolism in muscle as a result of loss of muscle mass; and (6) increased renal production of ammonia secondary to the respiratory alkalosis commonly seen in these patients, which is the result of primary hyperventilation [80] and hypokalaemia [81].
Cerebral uptake of ammonia is increased in patient with cirrhosis [82]. The blood–brain barrier remains anatomically intact in these patients but 13NH3 PET studies have shown that the permeability surface area to ammonia is increased [82].
There is no urea cycle in the brain. Ammonia is detoxified, in astrocytes, by the synthesis of glutamine through amidation of glutamate via glutamine synthetase. Once inside the brain ammonia exerts deleterious effects at many levels but particularly on astrocytes. These cells proliferate and exhibit significant changes in morphology, characterized by development of enlarged pale nuclei, prominent nucleoli, peripheral margination of chromatin and accumulation of glycogen. These changes, which are referred to as Alzheimer type II astrocytosis, are most prominent in the cerebral cortex, basal ganglia and cerebellum. Ammonia also has direct effects on cortical neurones, which affect postsynaptic inhibitory potentials [83], and the activity of the tricarboxylic acid cycle [84].
Nevertheless, despite the obvious importance of ammonia in the pathogenesis of hepatic encephalopathy, the correlation between circulating blood ammonia concentrations and neuropsychiatric status is poor. This reflects, at least in part, the technical difficulties associated with its measurement, and the differences in blood and brain ammonia concentrations which can be explained by variation in the compartmental pH. Indeed, the pH-dependent partial pressure of gaseous ammonia in arterial blood correlates more closely with the clinical and neurophysiological changes observed than plasma ammonia concentrations [69]. The correlation between neuropsychiatric status and glutamine is better. CSF levels of glutamine increase as the degree of neuropsychiatric impairment increases [70], as does the height of the glutamine/ glutamate signal observed on cerebral 1H-MRS [19].
Patients with congenital defects of one or more of the urea cycle enzymes may develop hyperammonaemia, and many features suggestive of hepatic encephalopathy including disturbed consciousness and Alzheimer type II astrocytosis [85]. However, they also develop seizures, which are rarely observed in patients with hepatic encephalopathy. Heterozygotes may develop hyperammonaemic coma following medication with sodium valproate.
Treatment that reduces the production and absorption of ammonia has a beneficial effect on patients with hepatic encephalopathy and is accompanied by reversal in the abnormalities observed on cerebral 1H-MRS [86].
Other gut-derived toxins implicated in the pathogenesis of hepatic encephalopathy include:
(1) indoles, produced by bacterial degradation of tryptophan [87]; (2) mercaptans, the sulphur-containing compounds responsible for foetor hepaticus [4]; (3) phenols, produced by bacterial degradation of phenylalanine and tyrosine; and (4) short- and medium-chain fatty acids. These compounds may have direct and independent effects on cerebral function or, more likely, act synergistically with ammonia.
Brain Water Homeostasis
The influx of excess ammonia into the brain results in accumulation of osmotically active glutamine within astrocytes. This results in astrocyte swelling. This is countered by efflux from the cell of other osmotically active compounds, principally myoinositol, but also taurine and α-glycerophosphorylcholine. The net result is the development of low-grade cerebral oedema. In vivo cerebral 1H-MRS studies in patients with cirrhosis and hepatic encephalopathy show a decrease in the myoinositol peak and an increase in the glutamine/ glutamate peak, supporting the concept of a disturbance in astrocytic volume homeostasis [88] (Fig. 8.7). The existence of low-grade cerebral oedema has also been demonstrated using cerebral magnetization and diffusion transfer MR imaging techniques [89,90] and by quantitative cerebral water mapping [91]. In addition, there is a good correlation between the changes observed on cerebral 1H-MRS and the severity of hepatic encephalopathy in patients with cirrhosis [88,92]; the changes are aggravated after TIPS insertion [88] and largely resolve following successful treatment [90] and liver transplantation [89] (Fig. 8.9).
Fig. 8.9. 1H-MR-spectroscopy water-suppressed spectra of an 8-mL voxel located in the parietal region in a patient with cirrhosis before (a) and after (b) liver transplantation, recorded with a stimulated echo acquisition mode pulse sequence (TR/TE, 1600/20 ms; acquisitions, 256). The main resonances correspond to N
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree