CHAPTER 6
5-aminosalicylates
Introduction
The aminosalicylates are a class of drugs used as the initial treatment to induce and maintain remission in patients with mild to moderate ulcerative colitis (UC). Sulfasalazine (SASP) was first developed in the 1940s as a treatment for rheumatoid arthritis and was the first aminosalicylate to be used for the treatment of inflammatory bowel disease (IBD). However, as many as 20–25% of patients were found to be intolerant or allergic to sulfasalazine, and thus the 5-aminosalicylic acid (5-ASA) agents were developed. These medications encompass a wide variety of preparations, each with a 5-aminosalicylate moiety that is responsible for its anti-inflammatory activity. Unmodified 5-ASA is readily absorbed in the stomach and duodenum; thus modification of 5-ASA is needed to target release of the 5-ASA moiety at the site of active inflammation in the small bowel and colon.
Preparations
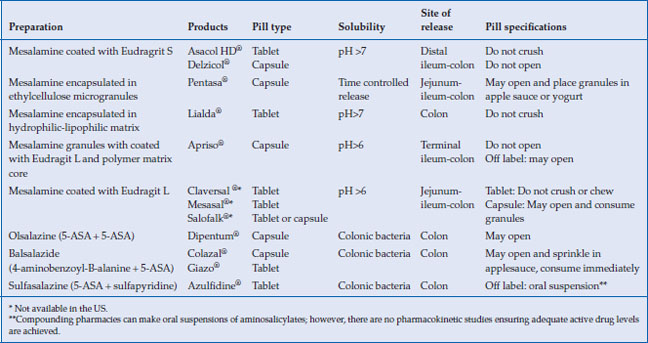
Many aminosalicylate preparations (oral or rectal administration) are approved for use in the United States, as well as other brands only available in Europe and Canada (Table 6.1). The 5-ASA family of medications includes: sulfasalazine (Azulfidine®), in which the 5-ASA is conjugated to sulfapyridine; mesalamine, in which the 5-ASA moiety is coated either with ethylcellulose (Pentasa®) or an acrylic-based resin, eudragit (Asacol HD®, Delzicol®); olsalazine (Dipentum®), in which two 5-ASA molecules are conjugated by an azo bond; and balsalazide (Colazal®, Giazo®), in which the 5-ASA moiety is conjugated to a 4-aminobenzoyl-beta-alanine carrier. Two once a day formulations of mesalamine are also available; one using a Multi-Matrix System (Lialda®) and the other with delayed release granules (Apriso®). Topical preparations of mesalamine are available in enema formulations (Rowasa®) as well as suppositories (Canasa®). Outside the US, mesalamine is marketed under a variety of other brand names, including Salofalk®, Claversal®, Ipocal®, Mezavant®, Mezavant XL®, Mesacol®, VEGAZ_OD®, Mesacron®, and Mesalazina®. Asacol® standard release (400 mg tablet) is no longer available in the US due to an inactive ingredient in its enteric coating material, dibutyl phthalate (DBP), which at high doses in animal studies caused malformations. Asacol HD® (800 mg tablet) does contain DBP and remains available.
Table 6.1 Properties of oral 5-ASA preparations
Clinical use and efficacy
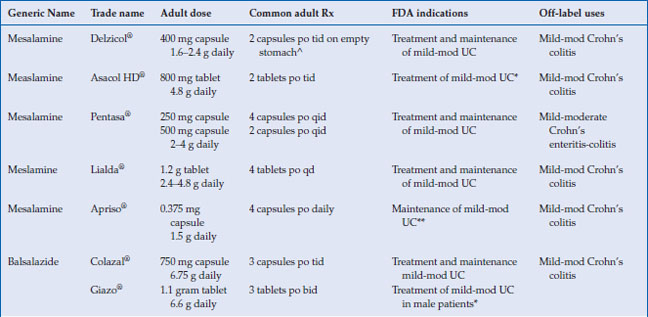
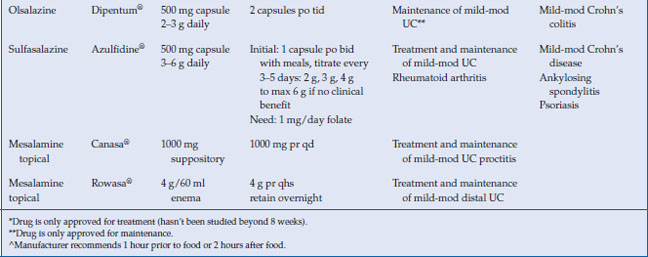
The 5-aminosalicylates are approved for the treatment and maintenance of remission of ulcerative colitis in adults, with the exception of Apriso® and olsalazine, which are only approved for maintenance in UC (Table 6.2). Mesalamine has been shown to be very effective in the treatment of mild to moderate active UC. Seventy-two percent of patients receiving 4.8 g/day of mesalamine in the ASCEND II trial achieved treatment success, which was either complete remission or clinical response at week 6. The same study showed 4.8 g/day mesalamine was superior in achieving endoscopic and histologic remission in active UC, with 48% of patients achieving remission by sigmoidoscopic index (p < 0.05), compared with 31% on placebo, and 39% of patients achieving microscopic remission, compared with 23% on placebo (p < 0.03). In the recent Cochrane meta-analysis, which include over 38 studies, there was no statistical difference in efficacy between once daily dosing 5-ASA and conventionally dosed 5-ASA. In addition when compared, 5-ASA was equal to sulfasalazine in inducing remission in mild to moderate UC.
Table 6.2 Commonly used 5-aminosalicylate drugs
5-ASA has also been shown to be effective in maintaining remission of UC in randomized controlled trials, with 12-month remission rates of 64% (38% for placebo, P = 0.0004). In the recent Cochrane meta-analysis, 5-ASA was effective in maintenance of clinical and endoscopic remission with a relapse rate of 41% for 5-ASA patients compared with 58% for placebo (RR 0.69, 95% CI 0.62–0.77). Oral 5-ASA administered once daily was as effective as conventional dosing for maintenance of remission in quiescent UC. Sulfasalazine was found to be superior to 5-ASA for maintenance of remission with 48% of 5-ASA patients relapsed compared to 43% of SASP patients (RR 1.14, 95% CI 1.03–1.27).
The 5-ASAs are not FDA approved for Crohn’s disease (CD), and their role in induction and maintenance of remission in patients with CD remains controversial. CD clinical studies suffer from heterogeneity of patients (disease locations, complications, previous surgery), varying doses of 5-ASA formulations, and primary endpoints using CDAI scores rather than endoscopic remission. In a pooled analysis of three clinical trials in active CD, Pentasa® 4 g/day was associated with a statistically significant reduction in CDAI compared to placebo (P = 0.04). Trials with other mesalamine formulations have shown between 45% to 55% clinical remission (CDAI<150), but none of them have used endoscopic remission as a primary endpoint. In the Cochrane meta-analysis, six RCTs examined the efficacy of 5-ASA in inducing remission in 910 active CD patients and found 68% randomized to 5-ASA did not achieve remission compared to 74% allocated to placebo, with a RR of 0.89 of not achieving remission with 5-ASA (95% CI = 0.80–0.99). This review also reported on two trials examining sulfasalazine in inducing remission in active CD and found 57% of patients randomized to sulfasalazine did not achieve remission compared with 68.9% for placebo, with a RR of 0.83 failure to achieve remission (95% CI = 0.69–1.00, p=0.05). Neither sulfasalazine nor mesalamine was effective in preventing relapse of CD. Despite this poor level of evidence, 5-ASA is commonly used off label for the treatment of Crohn’s disease.
Pharmacology: preparations and dosing
Delayed-release mesalamine, Asacol HD® and Delzicol®, has an acid resistant acrylic resin that allows encapsulation of oral mesalamine, which releases the active drug when the luminal pH rises above 7 in the terminal ileum and colon (Table 6.1). Pentasa® has ethylcellulose microspheres that initiate the release of 5-ASA to the small intestine and colon. These two 5-ASA formulations are used off-label to treat Crohn’s ileitis. Sulfasalazine, olsalazine, and balsalazide deliver 5-ASA predominately to the colon, since colonic bacteria are required to reduce the inactive parent drug to active 5-ASA in the colon. Both Lialda and Apriso have a pH-dependent release and thus target their release in the colon. All mesalamine preparations, including tablets, granules, and pellets, appear to be equally effective in inducing remission in active disease. Giazo was not found to be effective for women in clinical trials, and thus is approved for use only in men.
The American College of Gastroenterology (ACG) guidelines recommend combination oral and topical 5-ASA therapy for mild-to-moderate active left-sided or extensive UC disease. The dosage of oral mesalamine is between 2.4–4.8 g/day in divided doses. The optimal dose of oral mesalamine is controversial. The Cochrane meta-analysis showed no statistical significant difference in clinical improvement between mesalamine 4.8 g/day and 2.4 g/day, but subgroup analysis indicated that patients with moderate disease may benefit from the higher dosing. Higher doses of mesalamine (4.8 g daily) have been shown to achieve higher rates of mucosal healing than lower doses. Most aminosalicylates can be started at their target doses with certain exceptions (Table 6.2 and 6.3). Sulfasalazine should be started gradually, and the dose increased as tolerated by 50–75 mg/kg per day to a target dose of 4–6 g/day. Patients should be supplemented with 1 mg folate daily while taking sulfasalazine. Clinical improvement is usually within 2–4 weeks.
Table 6.3 Pediatric dosing of 5-aminosalcylates
| Generic name | Trade name | Pediatric dose |
| Mesalamine | Pentasa® | 50–75 mg/kg/day divided in two to three doses max: 4 g/day |
| Balsalazide | Colazal® | Children 5–17 years: 2.25 g po TID (three 750 mg capsules TID) or 750 mg po TID (one capsule TID) |
| Olsalazine | Dipentum® | 25–35 mg/kg/day divided in two to three doses |
| Sulfasalazine | Azulfidine® | Mild: 40–50 mg/kg/day divided every 6 hours Mod-severe: 50–70 mg/kg/day divided every 4–6 hours, do not exceed 4 g/day Maintenance: 30–50 mg/kg/day divided every 4–8 hours, do not exceed 2 g/day |
| Mesalamine topical | Canasa® | 1000 mg suppository pr qhs |
| Mesalamine topical | Rowasa® | 4 g/60 ml enema pr qhs |
| Only sulfasalazine and balsalazide are FDA approved for pediatric ulcerative colitis. | ||
For the maintenance of remission in distal UC, the ACG guidelines recommend topical mesalamine as an alternative to oral or combination therapy. Suppositories are used to treat proctitis up to 10 cm, while enemas can reach to the splenic flexure. In a meta-analysis of nine studies, rectal 5-ASA was effective for maintenance of both clinical and endoscopic remission over 6 months. The optimal dosing regimen of mesalamine suppositories and enemas has not been established, though studies have demonstrated that topical mesalamine preparations given as infrequently as three times weekly are effective in maintaining remission.
Mechanism of action
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






