Pathology
Dysplasia
The development of HCC is a process that takes place over many years. However, most of the changes occur at a genetic level, and these have no morphological counterpart. Several nodular anatomical lesions have been identified, which have an increased prevalence in livers containing hepatocellular carcinoma. Therefore, these lesions, which include cirrhotic nodules, dysplastic foci and dysplastic nodules, have been considered as possible precursor lesions for malignancy [30,31].
A dysplastic focus is a recognizable cluster of hepatocytes that is different from the surrounding liver. The cells within the focus differ from those of adjacent hepatocytes with respect to cytoplasmic staining, nuclear size, nuclear atypia and often hepatitis B surface antigen deposition. Dysplastic lesions are called foci if smaller than 1 mm in diameter and nodules if at least 1 mm in diameter.
Dysplastic nodules occur in a quarter of explanted cirrhotic livers, often coexisting with hepatocellular carcinoma [32]. These are commonly detected by ultrasound examination as nodules larger than the background cirrhotic nodules. Grossly dysplastic nodules often bulge above the cut surface and have a soft texture. Lesions may be either more bile-stained or paler than surrounding liver (due to fat). Necrosis and haemorrhage are not seen.
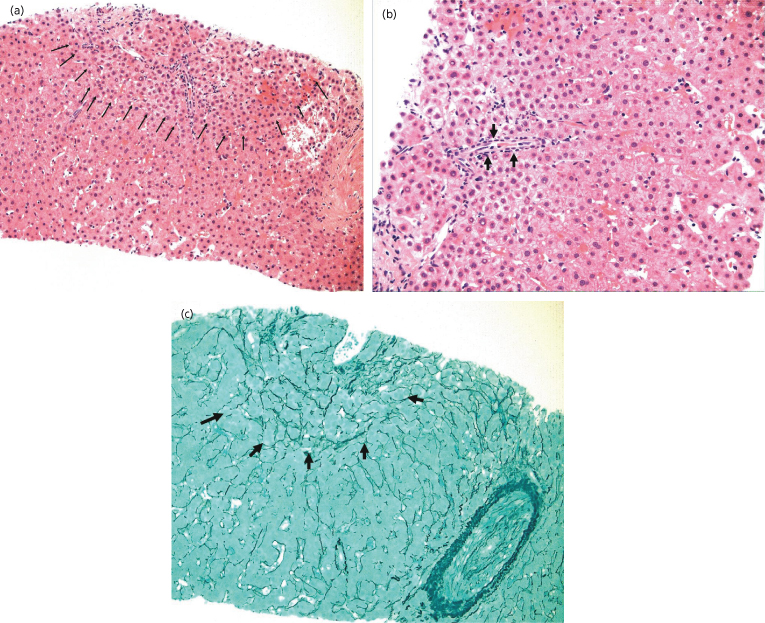
Histologically, low-grade and high-grade dysplastic nodules represent parts of a spectrum. Low-grade dysplastic nodules are comprised of hepatocytes that are minimally abnormal. They often have a fibrous surrounding scar, and are thus usually distinct from the surrounding cirrhotic liver. There may be increased cell density but there is no cytological atypia, except that the cells might be larger than non-nodular hepatocytes (large-cell dysplasia) [33]. High-grade lesions have more atypia and may be difficult to distinguish from carcinoma. High-grade dysplastic nodules may also have a nodular appearance, either vaguely nodular or with a more distinct margin. However, they do not have a true capsule. The cytoplasm may be eosinophilic or contain fat. There is some cellular atypia, in that the cells are usually small, and may show some minor changes in the nucleocytoplasmic ratio, or some nuclear pleiomorphism, but not enough to identify the lesion as malignant. There may be increased cell density and an irregular trabecular pattern. Unpaired arteries are frequently present in small numbers and portal tracts are present [34]. The liver cell plates are one to two cells wide (Fig. 35.2).
Fig. 35.2. High-grade dysplasia. (a) Lesion showing the boundary between normal liver and dysplastic nodule (arrows). (b) There is a single unpaired artery (arrow). Note the smaller (dysplastic) cells surrounding the artery compared to the cells are the other end of the biopsy, which are normal. The dysplastic cells replace the normal cells in the liver chords, which are intact. (c) The reticulin stain confirms that the chord structure is intact (arrows). (Courtesy of Dr. M Guindi)
Small-cell dysplasia has characteristics that suggest the condition may be a true precursor to HCC [35]. Firstly, there may be expression of proliferation markers such as proliferating cell nuclear antigen (PCNA), increased labelling index of silver staining of the nucleolar organizing region (AgNOR) and expression of Ki-67 [36], which is often highly expressed [37,38]. Secondly, transitions have been described between small-cell dysplasia and HCC, with the so-called nodule-in-nodule appearance [34,37], which is the appearance of a small focus of HCC within a larger dysplastic nodule. Based on a number of studies, the lesions can be ranked in ascending order of intensity of proliferation. Large cirrhotic nodules show least evidence of proliferation, similar to that seen in smaller cirrhotic nodules. Lesions showing large-cell dysplasia also have low proliferation indices and are not preneoplastic, and small-cell dysplasia shows proliferation markers similar to HCC.
Very Early HCC
Japanese pathologists have described what is probably the earliest discernable lesion of HCC, the so-called vaguely nodular lesion, which has ill-defined margins. This is also known as very early HCC [38,39], and has a somewhat vague outline on ultrasound. The cells show varying grades of dysplasia (Fig. 35.3) and more than 40% of the lesions contain fat. There may be invasion of the portal space by hepatocytes, but vascular invasion is absent. The pathology of these ‘very early HCC’ lesions has been defined in resected specimens, and therefore their natural history is unknown. However, small foci of typical HCC within the vaguely nodular lesions have been described, suggesting that these lesions are precursors of typical HCC. Table 35.3 indicates the differences between very early HCC and well-differentiated HCC. Very early HCC is probably the earliest morphological manifestation of malignancy. Histological examination shows the acinar cords being occupied by dysplastic hepatocytes. In resected specimens, a clear margin can often be seen within the cords, where normal hepatocytes are replaced by abnormal hepatocytes (Fig. 35.3). The first malignant cells start to destroy the normal architecture of the liver and also start to acquire their own arterial blood supply, as demonstrated by the appearance of a few unpaired arterioles. Portal tracts may still be present, but may be reduced in number, resulting in an overall reduced blood supply. Thus on dynamic imaging studies these lesions may be hypovascular, rather than the typical hypervascularity that characterizes well-differentiated HCC. The abnormal hepatocytes frequently contain fat and may show some degree of dysplasia, with altered nucleocytoplasmic ratios. In resected specimens, hepatocytes can be seen invading the portal tract (stromal invasion). The lesions may stain positively with CD34, a marker of vascular endothelium, indicating neoangiogenesis. They may also stain positively for glypican 3, a marker of early HCC [40]. Since all these studies have been performed with resected specimens it is not possible to say for certain that these lesions would have gone on to become clearly malignant. However, stromal invasion of the portal tract suggests at least some malignant potential [41]. In addition, follow-up of these lesions using ultrasound has demonstrated that some have become clearly defined malignancies.
Table 35.3. Characteristics of small nodular lesions in a cirrhotic liver
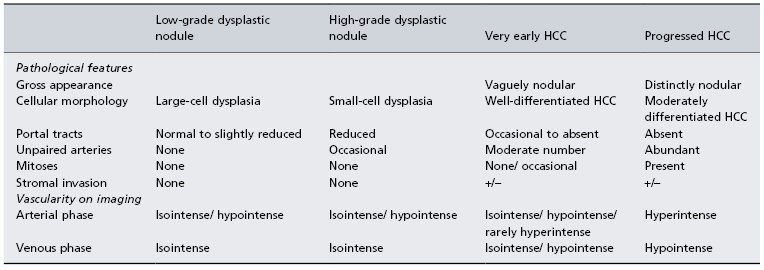
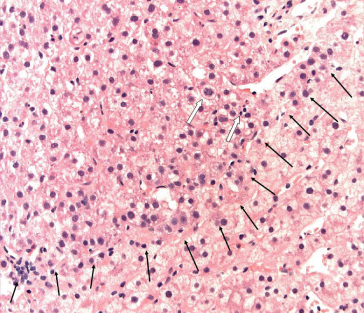
Fig. 35.3. Very early HCC. The interface between normal liver tissue and the very early HCC is outlined (black arrows). The cells of the HCC are dysplastic, but the presence of mitosis (white arrows) makes this likely to be HCC rather than high-grade dysplasia. (Courtesy of Dr. M Guindi)
Progressed HCC
Progressed HCC encompasses all histological variants of HCC apart from very early HCC. Progressed HCC can be categorized as well-differentiated, moderately differentiated and poorly differentiated, according to the presence or absence of cord-like structure, pseudoacinar formation and cellular morphology. Well-differentiated HCC consists of plates of cords of cells lined by endothelium. The cords may be many cells thick. The cells are hepatocyte-like but have vesicular nuclei, nuclear pleiomorphism and finely granular cytoplasm. Bile canaliculi may be seen. There is usually little or no fibrous stroma. Moderately differentiated tumours contain glandular structures, called pseudoacini, which may be bile stained. In poorly differentiated tumours the plate structure may be absent. The cells may be very large, with multiple bizarre nuclei. The most poorly differentiated tumours may be difficult to distinguish from other adenocarcinomas.
Pathogenesis
Although the relationship between chronic liver disease and HCC is clear, not much else is known about the pathogenesis of HCC. In the case of viral hepatitis, presumably, repeated episodes of necrosis and regeneration result in an increased DNA mutation rate and a decreased rate of repair of these mutations. Over time, sufficient specific mutations accumulate that lead to the development of HCC. However, in diseases such haemochromatosis and α1-antitrypsin deficiency, in which necrosis is not a major feature, it is more difficult to understand how HCC might develop. One theory is that cells affected by an accumulation of iron or α1-antitrypsin globules are injured, but not dead [29]. They nonetheless give off regenerative signals to neighbouring cells which consequently divide and would thus be subject to the same risk of mutations as in viral hepatitis.
HCC is a very vascular cancer. New growth of arterial vessels occurs early in pathogenesis. This implies a role for angiogenic factors, such as VEGF and its receptor. Many HCCs have high levels of VEGF expression [42,43], and high levels of VEGF, whether in tissue or in serum, may be correlated with poor outcome [42,43].
Microarray studies using gene chips have elucidated the pattern of gene expression in HCC [44–51]. There are at least three major patterns. Approximately one-third of tumours have abnormalities in proliferative signals related to the tyrosine kinase receptor pathway. These include EGFR, IGF-IR, RAS, and RAF/MAP-K pathways, as well as PI3K-Akt-mTOR pathways [44]. Another one-third of HCC have cell proliferation driven by activation of the Wnt/β-catenin pathway, mostly as a result of mutations in the β-catenin gene [46]. Yet another subset of HCCs has changes in genes involved in interferon signalling [44]. These results suggest that HCC is not a homogeneous disease, but that there are at least three, and maybe more, pathogenetic pathways that might lead to the phenotypic expression of HCC. The implication is that these different pathways may have different outcomes, both when treated and if left untreated. A separate gene signature has been associated with early recurrence of HCC after treatment [46,47]. Similarly, other gene signatures, associated with a poor prognosis, have been identified [45]. Proliferative and antiapoptotic genes are over-expressed in HCC with a poor prognosis.
Microarray analyses have also been applied to gene expression in normal liver, cirrhotic nodules, dysplastic nodules, early HCC and well-differentiated HCC [48–51]. These studies showed a differential pattern of gene expression between all the stages of disease. The studies confirmed that the usual biomarkers used for HCC surveillance (α-fetoprotein (AFP), glycosylated AFP (AFP-L3) and des gamma carboxyprothrombin (DCP)), are not expressed in early HCC. Glypican-3 in contrast, is expressed in early HCC but not in dysplastic nodules. This is the basis for the use of this marker as a histological stain to aid identification of early HCC.
Genome-wide microarray analysis has also identified a gene signature from tissue surrounding the malignant nodule that predicted late recurrence of HCC (more than 2 years after resection) [52]. This analysis also identified an expression pattern that predicted poor outcomes. There was some overlap between these two sets of markers, suggesting that in part the poor outcomes were related to recurrence. Late recurrence is thought to represent newly developed HCC, rather than recurrence from a previously treated HCC. That a gene signature can predict the onset of recurrent disease distant in time and location from the original tumour suggests the existence of a ‘field defect’, that is the whole liver may be abnormal, ‘preneoplastic’ and consisting of a single clone of cells. This also raises the possibility that in future biopsy of a cirrhotic liver might predict who is at risk for HCC and who is not.
Despite all these advances, the sum of genetic changes required to produce HCC remains uncertain.
Clinical Presentation
Symptomatic Presentation
If patients present with symptoms the cancer is advanced, and cure is unlikely. Patients presenting with symptoms may have any combination of jaundice, ascites or variceal bleeding; encephalopathy is rare. These symptoms may also develop as HCC progresses, in patients in whom previous treatment for HCC has failed. Thus it is important in all patients who present with these features to image the liver. Rupture of an HCC or bleeding into an HCC both cause severe abdominal pain. In addition, rupture may be associated with hypotension and shock. Finally, the first presentation of HCC may be with weight loss and other constitutional symptoms that are common to many cancers.
HCC has the unusual distinction of frequently producing paraneoplastic syndromes. These include hypoglycaemia, related to the production of an insulin-like peptide [53], hypercalcaemia [54], thrombocytosis [55] and hypercoagulability with venous thrombosis. New onset of any of these conditions in a patient with liver disease requires imaging of the liver.
Asymptomatic Presentation
More patients are being diagnosed at early stages of disease, before the onset of symptoms, due to more frequent imaging of the abdomen, usually by ultrasound, liberally used for a variety of abdominal complaints unrelated to liver disease. In addition, many patients known to be at risk for HCC are being diagnosed by the deliberate institution of surveillance programmes.
Differential Diagnosis
The differential diagnosis depends on the presentation. Patients with known chronic liver disease presenting with liver failure may not have HCC, and should be evaluated for other complications such as renal failure or infection, or other factors that may have precipitated symptoms. Patients presenting with rupture of an HCC should be evaluated as for any acute abdomen. Patients with liver disease presenting with cancer symptoms may have other intra-abdominal or haematological cancers. However, patients who present without symptoms who have a mass in the liver need to have HCC distinguished from benign lesions such as haemangioma, focal nodular hyperplasia, hepatic adenoma, metastases or other primary liver cancers. In most cases radiology is sufficient for diagnosis, but in some cases, as discussed later, a biopsy is needed.
Surveillance
The purpose of surveillance is to detect HCC early, at a stage where treatment responses are more durable and cure is more frequently possible. There is a single randomized controlled trial from China that compares survival in a screened versus unscreened population, in patients with chronic hepatitis B and offered only resection as treatment [56]. Despite several methodological flaws, such as a poor compliance, this study demonstrated a 37% decrease in HCC mortality in the screened group. It is not certain that these results can be directly applied to HCC related to other causes, or in other areas of the world. In particular, the likelihood of resection in patients with hepatitis C and HCC detected by surveillance is lower, because advanced cirrhosis is more frequent. Nonetheless, these data strongly suggest that surveillance is effective for enhancing survival.
In the absence of experimental data, cost–efficacy analyses have been used to evaluate whether HCC surveillance is effective. These confirm that surveillance of HCC in patients with cirrhosis due to hepatitis C or other causes is effective and cost effective [57–61]. Table 35.4 summarizes these studies. The results have also defined the HCC incidence rates at which surveillance becomes effective (providing more than 3 months increased life expectancy) and cost-effective. Therefore surveillance for HCC should be standard care for all patients with cirrhosis, and in hepatitis B for patients over age 40 for men and over age 50 for women [62]. Patients with a first-degree relative with HCC, patients coinfected with hepatitis B and HIV are at high risk, and should undergo surveillance when diagnosed. African men seem to acquire HCC at a younger age and should also start surveillance at an earlier age.
Table 35.4. Cost–efficacy analyses of hepatocellular carcinoma surveillance
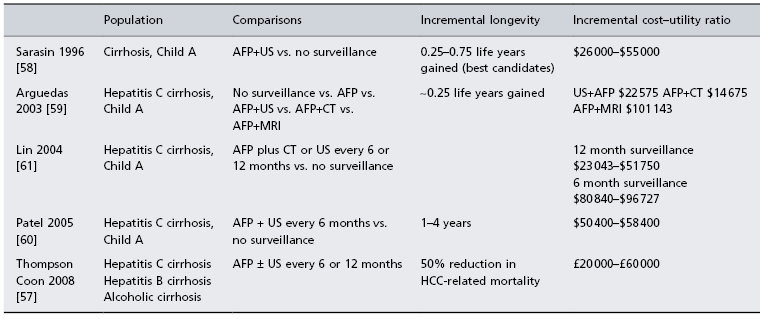
All cost efficacy analyses are only as good as the assumptions that are used to create the model. In the case of HCC surveillance, a lack of data means that there are many questionable assumption which can be faulted. The studies by Arguedas et al. [59] and Lin et al. [61] use data on the performance characteristics of CT scanning from studies in which the technique is used for diagnosis. There are no studies in which the performance characteristics of CT scanning have been determined when used as a surveillance test. The sensitivity and specificity are likely to be less good when used for surveillance. In the study by Thomson Coon et al. they assume that the sensitivity of AFP is not affected by tumour size. AFP increases as tumour size increases in those who are AFP-positive. AFP is more likely to be elevated in advanced disease, which disqualifies it as a useful marker for early disease. The analysis by Patel et al. [60] assumes that ultrasound cannot find lesions smaller than 2 cm. However, this is not the case, since in surveillance programmes up to 60% of HCCs are detected smaller than 2 cm. AFP, α-fetoprotein.
Surveillance Using Biomarkers
α-fetoprotein (AFP) [63,64], des gamma carboxyprothrombin (DCP) [65–67] and the AFP-L3/AFP ratio [66,67] have been proposed as surveillance tests for HCC. AFP-L3 is a glycosylated form of AFP which is produced in higher concentration by HCC than by normal liver. However, all these markers are more likely to be elevated in patients with advanced HCC than in patients with early disease [68,69]. Several studies have shown that AFP is both insufficiently sensitive or specific to be used as a surveillance test [11,63]. The other markers are less well studied, but the initial results are not promising. Therefore these tests should not be used for HCC surveillance [62].
Surveillance by Ultrasonography
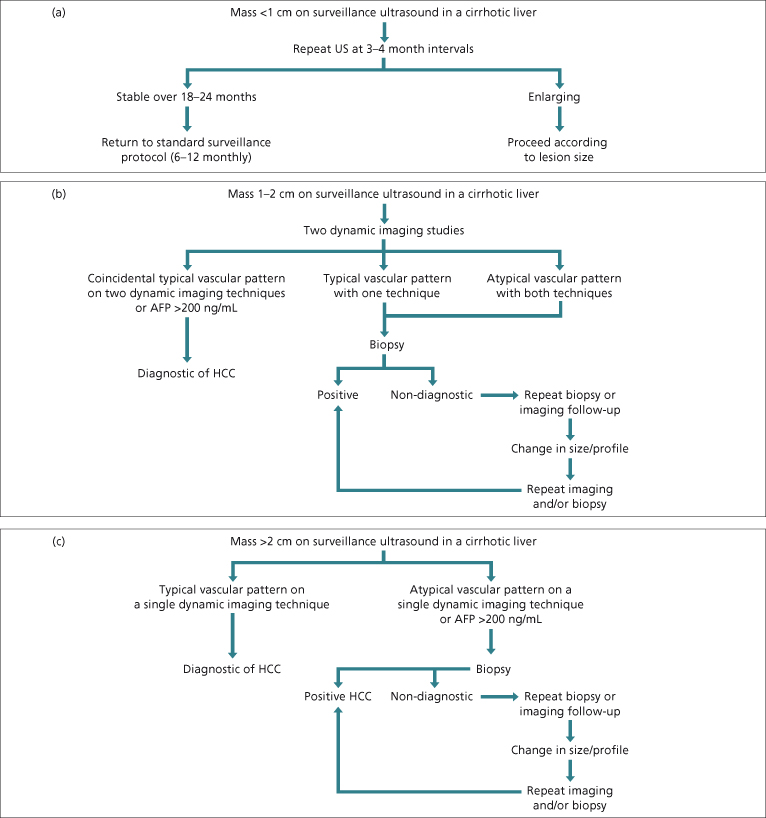
Ultrasonography is the surveillance test of choice for HCC [62]. Despite the presence of cirrhosis, ultrasonography can identify nodules that are either HCC or dysplastic nodules that may become HCC. Most cirrhotic nodules are smaller than 1 cm, although larger nodules are not uncommon. Therefore, any nodule that is larger than 1 cm or that enlarges over time is suspicious for HCC and warrants investigation using the recall algorithms presented in Fig. 35.4.
Fig. 35.4. Diagnostic algorithms for HCC at different sizes. These algorithms were developed for patients with cirrhosis but apply equally well to non-cirrhotic patients with chronic hepatitis B or C. However, they do not apply to patients with no underlying liver disease, in whom all small (<3 cm) lesions suspicious for HCC should undergo biopsy. (a) Diagnostic algorithm for lesions smaller than 1 cm. These have a low likelihood of being HCC so the algorithm suggests only monitoring. (b) Diagnostic algorithm for lesions between 1 and 2 cm in diameter. The likelihood of these being HCC is higher than for lesions smaller than 1 cm. However, radiology can be difficult to interpret at this size lesion; therefore the recommendation is that two diagnostic radiological imaging tests are required for a positive radiological diagnosis. (c) Diagnostic algorithm for lesions larger than 2 cm. Typical radiological appearances are highly specific, so if present no biopsy is necessary. AFP, α-fetoprotein.
Surveillance Interval
The surveillance interval depends on the growth rate of the tumour and on the size at which outcome changes. HCC doubling time ranges on average from 4 to 12 months [70,71]. The prognosis of HCC starts to decline once the HCC is larger than about 2 cm in diameter [72,73]. Thus ideally, the surveillance interval should be set so that lesions will be detected before they reach 2 cm in diameter. The ideal surveillance interval is not known. Several studies have suggested that there is no difference in outcome with 6 versus 12-monthly surveillance [74,75]. However, more recent data suggest that survival is better following a 6-monthly surveillance interval [76]. Thus, the recommendation is to provide surveillance every 6 months [62].
Diagnosis
The tests used to diagnose HCC include radiology, biopsy and AFP serology. Which tests should be used depends on the clinical context. Imaging, either with CT scan or MRI, is always required to determine the extent of disease. In the setting of a patient with known hepatitis B or cirrhosis of other aetiology when a mass is found incidentally during surveillance by ultrasound, the likelihood of it being HCC depends on its size. The larger the lesion, the more likely it is HCC.
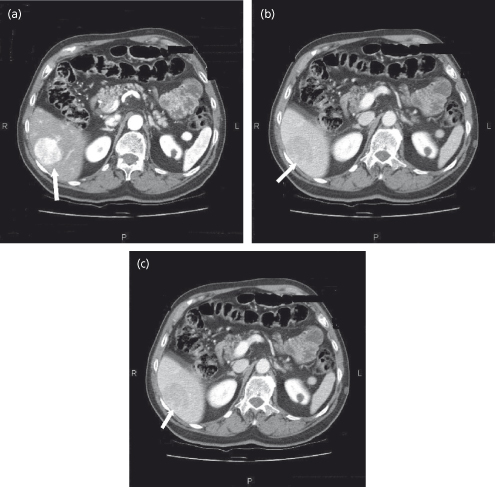
The radiological features of HCC are highly specific [77,78]. However, the smaller the lesion, the less likely that that typical features will be found. HCC exhibits hypervascularity on the arterial phase of a dynamic study (CT, MRI or contrast ultrasound), and ‘washout’ during the venous phase. The physiological basis for these appearances is as follows. During the arterial phase the portal venous blood in the liver dilutes the contrast agent, which is in the arterial blood. The tumour is fed by only arterial blood so that the contrast is undiluted. Therefore the tumour contains a higher concentration of contrast agent, and appears brighter than the surrounding liver. During the venous phase the portal blood now contains contrast, whereas the arterial blood feeding the tumour no longer contains contrast. Thus the liver will be brighter than the lesion, or, in the terminology used, the lesion exhibits ‘washout’ of contrast (Fig. 35.5). Other vascular tumours tend to have a dual arterial and venous blood supply, so they may enhance more than the liver in the arterial phase—they do not ‘washout’. This has led to the development of diagnostic algorithms, which define the role of radiology and tumour biopsy (Fig. 35.5)[62].
Fig. 35.5. Triphasic CT scan of HCC. (a) The arterial phase is identified by the aorta, which is very bright. The HCC in the liver is brighter than the surrounding liver (arrow). (b) In the venous phase (identified by a weaker signal in the aorta) the HCC is less bright than the surrounding liver (washout) (arrow). (c) In the delayed phase, the HCC remains less bright than the surrounding liver.
If the AFP is greater than 200 ng/mL in the setting of a mass in a cirrhotic liver, the likelihood of HCC is greater than 90%, and biopsy is not required [79].
A new lesion detected with surveillance ultrasound is an indication for additional investigation. The strategy of waiting to see whether the lesion will grow and then treat as malignancy runs the risk of delaying treatment until the lesion reaches a size where the likelihood of complete eradication of tumour decreases [80]. For example, if a lesion is detected at 1 cm, then 6 months later it may be larger than 2 cm, and less likely to be completely eradicated.
Lesions less than 1 cm in diameter on ultrasound in a cirrhotic liver have a low likelihood of being HCC [81]. Even if CT or MRI show arterial vascularization, the vascularized areas may not correspond to HCC foci [82,83]. However, the possibility remains high that minute hepatic nodules detected by ultrasonography may become malignant over time [84]. Therefore these nodules need to be regularly followed-up every few months in order to detect growth suggestive of malignant transformation (Fig. 35.4a). Lack of growth over a period of more than 1–2 years suggests that the lesion is not HCC.
Lesions between 1 and 2 cm have an indeterminate likelihood of being HCC. The AASLD guidelines [62] recommended that the diagnosis of HCC can be made without biopsy in patients with chronic liver disease and cirrhosis who have a mass between 1 and 2 cm if the mass shows characteristic radiological features on at least two dynamic imaging techniques (Fig. 35.4b). Lesions showing typical features on both techniques should be treated as HCC since the positive predictive value of the clinical and radiological findings exceeds 95% [77,78]. If the two techniques give discordant results (one typical and one atypical) or if both techniques give atypical results, a biopsy is required to confirm the diagnosis. If the lesion is larger than 2 cm, only a single dynamic radiological study is necessary to confirm the diagnosis if the findings are typical of HCC (Fig. 35.4c). If the appearances are not typical, a biopsy should be done. It is important to note that although a positive biopsy is diagnostic, a negative biopsy can never be taken as proof that an HCC does not exist. In these small lesions, placement of the biopsy needle is difficult and sampling errors are common. Pathological interpretation of the earliest changes of HCC is also difficult and the difference between dysplasia and HCC may be subtle and easily missed. Thus, a negative biopsy requires either a second biopsy or more frequent follow-up with ultrasound. If the lesion either enlarges or changes appearance, the diagnostic work-up must be repeated.
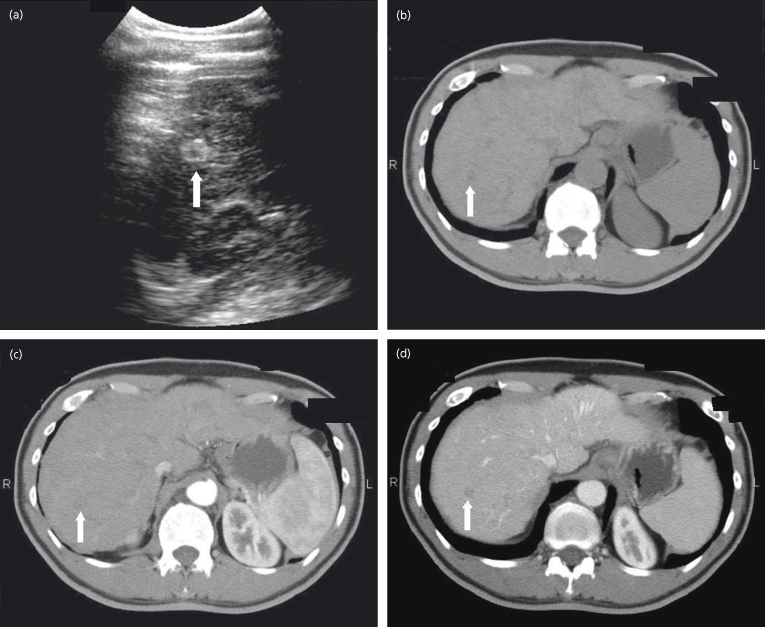
Very early HCC is hypovascular on imaging, and has ‘atypical’ imaging (Fig. 35.6); these lesions should be biopsied. Cholangiocarcinoma is vascular in the arterial phase but does not washout, so a biopsy would be indicated.
Fig. 35.6. Very early HCC on ultrasound and triphasic CT scan. Note that the lesion is hypoattenuating (less bright) in the non-contrast phase because of fat in the lesion. In the arterial and venous phases it remains hypoattenuating indicating that it is relatively hypovascular compared to the surrounding liver.
Role of Liver Biopsy
Liver biopsy to diagnose HCC remains a controversial issue. Biopsy carries a median risk of tumour seeding of 2.29% (0–11%) [85] so if resection or transplantation is contemplated, tumour seeding converts a curable situation to an incurable one. The risk of tumour seeding from biopsy of small lesions is less well characterized but when the lesion is small, seeding is reduced if ablative therapy is used after biopsy (median risk is 1.5% for percutaneous ethanol injection (PEI) or radiofrequency ablation (RFA)) [85]. Biopsy is indicated when radiology is unable to confirm a diagnosis. The biopsy has to be performed under ultrasound guidance, and should be with as large a needle as is safe to use. This ensures a larger specimen, increasing the diagnostic accuracy. A core biopsy is required. A fine-needle aspirate cannot distinguish well-differentiated HCC from normal hepatocytes, as architectural features are used to distinguish a well-differentiated HCC from normal tissue, such as thickened cords, trabecular pattern, etc. These features cannot be assessed in fine-needle aspirate specimens [85].
Staging of HCC
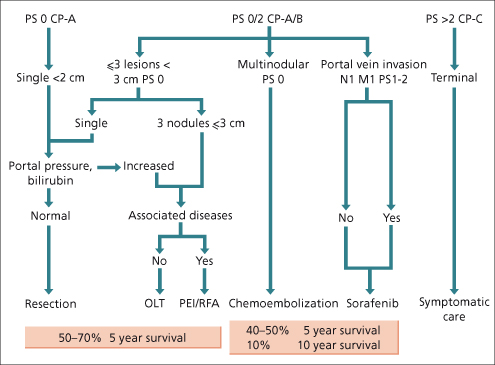
There are many staging systems for HCC; none are universally accepted. In Europe and North America, the Tumour Node Metastasis (TNM), Barcelona Cancer of the Liver Clinic (BCLC) [86] (Fig. 35.7) and Model for End Stage Liver disease (MELD) [87] or Cancer of the Liver Italian Program (CLIP) [88] systems are in common use. TNM is the classical cancer staging system, but is of less value for HCC because it does not capture liver function. Since Child–Pugh status is one of the best predictors of outcome of HCC, this is a major omission. An attempt to correct this is the sT or simplified TNM [89]. This includes an assessment of fibrosis. TNM also includes vascular invasion, including microvascular invasion, as a prognostic criterion. This information is not usually available prior to resection or transplantation, again limiting the usefulness of this staging system. MELD was not designed as an HCC staging system, and should not be used as such. The BCLC staging system was developed to separate out clinical stages of HCC that have distinctly different outcomes. It includes measures of liver function and performance status, both significant predictors of outcome. The BCLC staging system also has the advantage that it links different clinical stages to treatments that are appropriate to that stage. The CLIP score was developed using classical methodology, but suffers from being insensitive to prognosis associated with small HCC. Tumour size does not change the CLIP score until the lesion occupies 50% of the liver.
Fig. 35.7. The Barcelona Cancer of the Liver Clinic (BCLC) scheme for staging of liver cancer and the treatment strategies that are recommended for each stage. The expected survival from the categories of treatment is shown below. PS, performance score; CP, Child−Pugh class; N, node; M, metastases; OLT, orthotopic liver transplant; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation.
The Chinese University Prognostic Index (CUPI) [90] was developed primarily in patients with hepatitis B, where as the CLIP and BCLC were developed primarily in hepatitis C. CUPI has not gained wide acceptance. Finally, in Japan, several additional staging systems have been developed, which are not used elsewhere. The discussion of treatment that follows is based mainly on use of the BCLC system.
The prognosis of HCC is largely dependent on the stage at presentation, and on the underlying liver disease. Child–Pugh stage is a major determinant of outcome, confirmed in a systematic review of outcomes in HCC [91]. Other important predictors of outcome are tumour stage (irrespective of staging system), age, tumour burden and vascular invasion and performance status. An elevated AFP is a predictor of poor outcome. Figure 35.7 gives approximate expected 5-year survival for patients with HCC classified by the BCLC staging system [86].
Treatment
Preamble
There are a large number of therapeutic techniques that have been used for HCC. These include liver resection, liver transplantation, hepatic artery ligation, hepatic artery embolization, chemoembolization, internal radiotherapy, external beam radiotherapy, various chemotherapy regimens, local ablation with heat (radiofrequency or microwaves), cold (cryotherapy), corrosive substances such as ethanol, acetic acid or hot saline, and more recently targeted agents such as raf-kinase or VEGF inhibitors. The level of evidence for most of the therapeutic options is limited to phase 1 or 2 cohort studies. As a result, treatment of HCC is not adequately standardized. There are few randomized controlled trials of HCC therapy, most of which are limited to the treatment of advanced disease. There are no studies that compare treatments considered effective for early stage disease (surgical resection, transplantation, percutaneous ablation) to no treatment or best supportive care, nor are such studies ever likely to be performed.
Resection
This is the treatment of choice for HCC in patients without cirrhosis, who account for just 5% of the cases in Western countries, and for about 40% in Asia. These patients will tolerate major resections with low risk of morbidity. Resection in patients with cirrhosis carries increased risks of hepatic decompensation with right lobectomy being more risky than left lobectomy. In properly selected patients, the postoperative mortality should be no higher than 1–2%, and in many centres it is lower. The 5-year survival after resection should exceed 50 to 70% [92,93].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








