The diseases are of unknown cause, they are commoner in twins and there is no chronicity.
Acute Fatty Liver of Pregnancy
The first description is usually attributed to Sheehan [10] who, in 1940, described obstetric acute liver atrophy as a cause of jaundice in pregnancy. It affects approximately 1 : 14 000 pregnancies [11].
Clinical Features
The onset is usually between the 34th and 36th week and is marked by nausea, repeated vomiting and abdominal pain followed by jaundice (Table 30.2). Earlier presentation is so exceptional that alternative diagnoses should be considered. It is commoner with twins and male births and in primiparae [9].
Table 30.2. Clinical and laboratory features of 12 patients with acute fatty liver of pregnancy
(data from [9])
| Feature | Number of patients |
| Nausea/ vomiting | 12 |
| Severe heartburn | 4 |
| Abdominal pain | 7 |
| Jaundice | 11 |
| Leucocytosis | 12 |
| Thrombocytopenia | 9 |
| Proteinuria | 7 |
| Oedema | 7 |
| Hypertension | 8 |
| Serum urea increased | 9 |
In those severely affected, the course is marked by encephalopathy, renal failure, pancreatitis, oesophagitis, haemorrhages, disseminated intravascular coagulation and pulmonary emboli. Ascites may be found.
Polydipsia and polyuria with transient diabetes insipidus have been reported [9,12]. Signs of pre-eclampsia are found in 50% [9]. The differential diagnosis lies between acute viral or drug induced hepatitis and toxaemia of pregnancy, including HELLP syndrome. Obstetric causes of renal failure, such as haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura, must also be considered—a blood film can help in the differential diagnosis, as well as careful attention to the antecedent history.
Serum Biochemical Changes
Serum ammonia and amino acid levels are increased, reflecting mitochondrial failure. This is also suggested by lactic acidosis. High serum uric acid levels are usual and may be related to the tissue destruction and lactic acidosis [9]. Plasma urea, uric acid and creatinine are usually elevated, even with mild jaundice. Uric acid concentrations rise days before symptoms [9].
Hypoglycaemia can be profound. Hyponatraemia and hyperkalaemia can occur.
Hyperbilirubinaemia is 90% conjugated, without haemolysis in contradistinction to pregnancy toxaemia where jaundice is rare except when there is haemolysis. Serum transaminase values are variable, rarely above 500 IU/L and may be normal [13]. Total serum gammaglobulin is not elevated and can help distinguish acute fatty liver from acute or chronic hepatitis [9].
Haematological Findings
Leucocytosis and thrombocytopenia are common but the blood film may be leucoerythroblastic [9].Prothrombin time and partial prothrombin time are increased. Fibrinogen levels are decreased. Severe bleeding is frequent, but disseminated intravascular coagulation is found in only 10% [9].
Liver Histology
Liver biopsy is not usually necessary, as often the obstetric management regarding delivery takes precedence over the precise diagnosis of the liver dysfunction, but can be performed by the transjugular route. The histological picture is of microvesicular and macrovesicular fat droplets with ballooned hepatocytes containing dense, central nuclei (Fig. 30.2). Zone 1 (periportal) is relatively spared. The microvacuoles may be clearly recognized only on fresh sections stained for fat with such methods as oil red O (Fig. 30.3) [9,13,14].
Fig. 30.2. Acute fatty liver of pregnancy. Hepatocytes have a foamy appearance with a central dense nucleus. (H & E, ×120.)
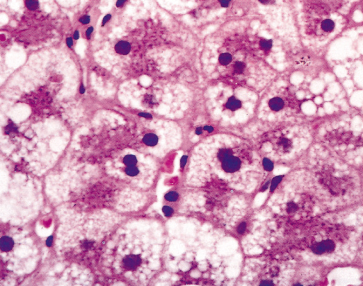
Fig. 30.3. Acute fatty liver of pregnancy: zone 3 hepatocytes are full of microvesicular fat droplets. Portal zones are normal and inflammation is minimal. (Oil red, ×40.)
Foci of inflammation and necrosis may be seen; also cholestasis with bile canalicular plugs and bile-stained Kupffer cells. Liver architecture is normal.
Electron microscopy confirms vacuoles and may show a honeycomb appearance in the smooth endoplasmic reticulum. Mitochondria are large and pleomorphic with paracrystalline inclusions [12].
Multiorgan involvement is shown by fatty infiltration of the renal tubules and renal lesions typical of pre-eclampsia. Fatty infiltration of the pancreas and the heart have been reported [13].
Ultrasonography of the liver may show a diffuse increased echogenicity which is very suggestive of acute fatty liver of pregnancy (AFLP). A normal sonogram does not exclude the diagnosis. CT shows a low attenuation value in 30% [15].
Course and Prognosis
Early recognition with prompt treatment has allowed diagnosis of milder cases and the current maternal mortality is 0–18% [16]. The fetal mortality (9–23%) remains high [16], in part related to inherited enzyme defects of long chain fatty acid metabolism described below.
Death is usually due to extrahepatic causes, such as disseminated intravascular coagulation with massive haemorrhage, including subcapsular haematoma and rupture and to renal failure. These features are not seen in the less-severe cases.
Recurrences are extremely rare and related in part to genetic predisposition [17].
Management
The management of the average, mild case is careful observation of the mother and fetus in hospital. If the mother’s status deteriorates (intractable vomiting, increased jaundice and features of a coagulopathy), the pregnancy should be delivered.
Coagulopathy, renal failure, hypoglycaemia and infections are treated. The prognosis is relatively favourable if intensive care is adequate [18]. Intra-abdominal haemorrhage may necessitate laparotomy for clot evacuation. The intensive care must continue post-partum and intra-abdominal haemorrhage may follow caesarean section. Hepatic transplantation is rarely necessary [18,19].
The baby may need corticosteroids to treat lung immaturity.
Oesophagitis with bleeding is a frequent complication [9] and omeprazole or a similar drug should be given.
Aetiology
AFLP can be regarded as a member of the mitochondrial cytopathy family (Table 30.3) [20]. Members include Reye’s syndrome, genetic defects in mitochondrial enzymes and drug reactions, especially to sodium valproate and nucleoside analogues.
Table 30.3. The mitochondrial cytopathies
| Causes |
| Acute fatty liver of pregnancy |
| Reye’s syndrome |
| Genetic defects in mitochondrial function |
| Drug-related |
| Features |
| Vomiting and apathy |
| Lactic acidosis |
| Hypoglycaemia |
| Hyperammonaemia |
| Microvesicular fat in organs |
Apart from the breakdown of carbohydrate, nearly all the reactions involved in energy production take place in mitochondria. Some oxidative phosphorylation includes the oxidation of fuel molecules by oxygen and simultaneous energy transduction into ATP. Fatty acids are broken down in the mitochondria into shorter, derivative fatty acids and acyl-CoA. This cycle of repeated fatty acid cleavage requires a series of specific enzymes.
The mitochondrial cytopathies are marked by vomiting and weakness. Lactic acidosis and metabolic acidosis are related to defective mitochondrial energy supply and defects in oxidative phosphorylation. Hypoglycaemia may be related to failure of mitochondrial citric acid cycle function. Raised blood ammonia relates to defects in mitochondrial Krebs’ cycle enzymes. Microvesicular fat is seen in the organs.
Several cases of AFLP are associated with homozygous long-chain 3-hydroxyacyl-coenzyme A dehydrogenase (LCHAD) deficiency in a fetus who has a heterozygote mother for LCHAD deficiency, whereby the latter cannot metabolize the extra free fatty acids that are not metabolized by the fetus [21,22]. However, several factors appear to contribute to this fetal–maternal interaction [21]. LCHAD deficiency is caused by a genetic defect of mitochondrial trifunctional protein [23]. Careful observation of children born to mothers with AFLP is warranted as they may be homozygous for LCHAD deficiency. These children are at risk of hypoglycaemia, fatty liver, dilated cardiomyopathy, progressive neuromyopathy and sudden infant death syndrome [24,25]; thus, early diagnosis and dietary intervention could be lifesaving. Fortunately, prenatal diagnosis is possible [26]. AFLP is also associated with HELLP (haemolysis, elevated liver enzymes, low platelet count) syndrome [27]. The G1528C mutation resulting in the conversion of glutamic acid to glutamine (E474Q), has been found to be present in 20% of AFLP cases [28]. Short-chain acyl-CoA dehydrogenase deficiency has also been associated with AFLP [29].
Pregnancy per se may affect mitochondrial function. The mode of initiation of the mitochondrial cytopathies, apart from the genetic enzyme defects, is uncertain. It might be viral, or due to drugs such as aspirin which has been implicated in the development of Reye’s syndrome. It might be toxic and AFLP has followed exposure to toluene [30]. Nutritional factors have also been suggested.
AFLP should be regarded as part of a systemic mitochondrial dysfunction affecting particularly liver, muscle, nervous system, pancreas and kidneys. The mother should be investigated for mitochondrial dysfunction, especially if the baby dies in utero or exhibits failure to thrive.
Pregnancy Toxaemias
These conditions are characterized by hypertension, proteinuria and fluid retention. The term ‘pregnancy toxaemia’ includes a spectrum of conditions (Table 30.4). The target organs are the uterus, kidney and brain. Hepatic damage is only seen in patients with severe pre-eclampsia and eclampsia.
Table 30.4. Spectrum of pregnancy toxaemias
| Pre-eclampsia |
| HELLP syndrome |
| Infarction |
| Bleeding and rupture |
The aetiology of pre-eclampsia is unknown. It is marked by generalized vasospasm with increased systemic vascular resistance and enhanced pressor responses to endogenous vasoconstrictors. Endothelial cell injury may decrease endothelial-dependent vasodilators and increase production of vasoconstrictors coming from both endothelial cells and platelets. Serum from pre-eclamptic patients contains factors that increase endothelial cell permeability [31]. The vascular endothelium may be a target for blood-borne products of reduced placental perfusion [32].
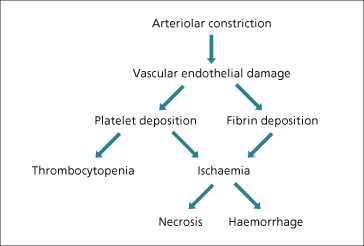
Vascular endothelial damage leads to platelet deposition, thrombocytopenia and fibrin deposition in sinusoids. The resultant ischaemia accounts for the focal and diffuse hepatocellular necrosis and haemorrhages in zone 1 (Fig. 30.4).
Fig. 30.4. The liver in eclampsia. Hepatocellular necrosis and haemorrhage follow ischaemia related to vascular endothelial damage.
In mild cases, increases in serum alkaline phosphatase and transaminase values are frequent [33]. Minor signs of disseminated intravascular coagulation, such as a reduction in platelets, are also common.
Jaundice is infrequent and often terminal. It is usually haemolytic with disseminated intravascular coagulation. Failure of renal bilirubin excretion may contribute. Serum bilirubin is less than 6 mg/dL (100 µmol/L).
Severe pre-eclampsia or toxaemia may present with epigastric pain, nausea, vomiting, hypertension, right upper quadrant tenderness due to infarction [34] or haematoma [35,36].
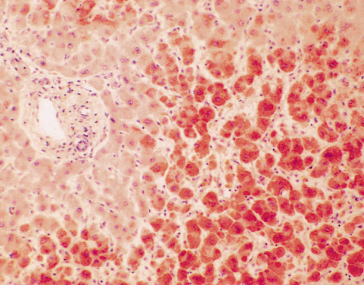
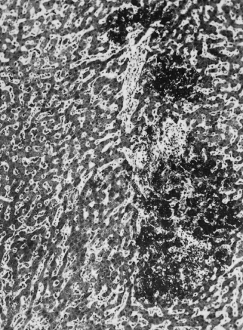
Hepatic histology shows periportal (zone 1) fibrin deposits [37] and haemorrhage, which progress to small necrotic foci, infarcts and haematomas. Zone 3 necrosis and haemorrhage represent shock. An inflammatory reaction is characteristically absent (Fig. 30.5). Capillary and hepatic arterial thrombi and, rarely, intrahepatic portal venous thrombi may be noted. Serum transaminases are usually more than 10 times elevated.
Fig. 30.5. The liver in eclampsia. Focal periportal necrosis of liver cells; the lesion contains fibrin. (Mallory’s phosphotungstic acid, ×80.)
Rupture of the liver is associated with shock and accounts for 15% of deaths [37].
Ultrasound and CT show focal filling defects, or haematomas.
The treatment of both mild and severe toxaemia is by delivering the pregnancy and by supportive care.
The HELLP Syndrome
This is a rare variant of pre-eclampsia [38,39]. It consists of haemolysis, elevated liver enzymes and low platelet count [40]. It often affects multipara. The blood pressure may be normal and proteinuria may be absent.
Liver histology shows fibrin deposition [41]. This suggests severe pre-eclamptic liver disease and calls for immediate delivery. The laboratory results do not reflect hepatic histology [41]. Perinatal mortality is 10–60% and the maternal mortality 1.5–5% [42].
Management is delivery of the fetus and supportive therapy for the mother, as for eclampsia. Rarely, liver transplantation has been used [43].
Women heterozygous for factor V Leiden have an increased risk of developing HELLP syndrome [44], while placental CD95 ligand (CD95L) has been shown to act systemically to cause liver damage in patients with HELLP syndrome, and blockade of CD95L results in reduced liver damage via inhibition of apoptotic mechanisms [45]. This finding may eventually lead to new therapies, although the relationship between HELLP syndrome and defects in fatty acid oxidation requires further evaluation [46].
Overlap between AFLP and Hypertension-Associated Liver Dysfunction of Pregnancy
Many patients with AFLP show signs of pre-eclampsia [9,47] (Table 30.5); therefore, the two conditions may form part of the same disease spectrum, or may be associated with the same metabolic defect, namely LCHAD deficiency. Indeed, both older [48] and newer [38] studies have found fatty livers in a significant proportion of patients with eclampsia. One study involving 41 consecutive patients with pre-eclampsia with or without liver dysfunction revealed that all women had a significant amount of microvesicular fat upon oil red O staining [49].
Table 30.5. Acute fatty liver of pregnancy and toxaemias contrasted: overlaps exist
| Acute fatty liver | Toxaemia | |
| Abdominal pain | 50% | 100% |
| Jaundice | 100% | 40% |
| Serum transaminases (× normal) | <10 | >10 |
| Scans | Diffuse change | Focal abnormalities |
| Liver biopsy | Microvesicular fat | Fibrin (perisinusoidal) |
| Liver failure | Present | Absent |
Although the association between AFLP and HELLP syndrome or pre-eclampsia is suggested by the frequency with which both conditions occur in mothers of infants who have LCHAD deficiency [27], this apparent connection may in fact be due to misdiagnosis of AFLP as HELLP syndrome [9,50]. Some sources recommend monitoring platelet counts, as these levels decrease just before HELLP syndrome and AFLP become manifest [51].
Hepatic infarcts, haematomas, and liver rupture in pregnancy are associated with severe pre-eclampsia or eclampsia in 80% of cases [52] and, to a lesser degree, with HELLP syndrome and AFLP [52], often in association with disseminated intravascular coagulation. Liver rupture presents as sudden abdominal pain associated with nausea and vomiting, and shock may develop very quickly [53]. The initial treatment of choice is the conservative management of hepatic haematomas; however, facilities for urgent laparotomy should be available. Arterial embolization and surgery are needed for rupture. Liver transplantation may be successful [54], but rarely needed.
Hepatic adenomas, often with peliosis hepatis, and often associated with oral contraceptives, may rupture during pregnancy (see Chapters 24 and 34).
Cholestasis of Pregnancy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








