Hepatitis A and B Vaccination in HIV Patients
All HIV patients should be tested for viral hepatitis and vaccinated against hepatitis A and B as early as possible if not immune (Table 22.1). HIV patients are less likely to respond to vaccination [2]. Response rates ranging from 17 to 56% have been reported in HIV patients, being especially impaired in those with lower CD4 counts [2]. No significant, distinctive, adverse clinical reactions to HBV vaccination have been described in HIV patients. Transient elevations in plasma HIV RNA lasting for several days to a few weeks have been sporadically reported [3]. The standard HBV vaccination schedule consists of three intramuscular doses of the hepatitis B vaccine at 0, 1 and 6 months. Hepatitis B vaccines are available as a single-antigen formulation and also in fixed combinations with inactivated hepatitis A vaccines. Anti-HBs titres should be checked 1 month after completion of the vaccine series to document response. If there is no response, revaccination can be considered.
Table 22.1. Approach to viral hepatitis in HIV patients
| Test required by all HIV patients | Recommendation |
| Hepatitis A: HAV IgG | If negative: vaccinate |
| Hepatitis B: HBsAg, anti-HBs, anti-HBc | If negative: vaccinate |
| If positive for HBsAg: manage as chronic HBV infection | |
| If only anti-HBc positive: check serum HBV DNA If HBV DNA negative: vaccinate against HBV If HBV DNA positive: manage as chronic HBV infection | |
| Hepatitis C: Anti-HCV | If positive: test for HCV RNA, and for HCV genotype if viraemic |
HIV individuals are less likely to maintain sustained high and protective anti-HBs titres [4]. Breakthrough HBV infections have been reported in HIV patients when a decline in anti-HBs concentrations to less than 10 mIU/mL occurred. It may be difficult to distinguish between waning immunity and non-response in individuals with an unknown anti-HBs response following HBV immunization. The degree of anti-HBs response 4 to 12 weeks after a single booster dose may differentiate the two antibody response patterns. True non-responders will not elicit serum anti-HBs or show a minimal rise in titre. In contrast, those with waning immunity generally have a robust anamnestic response. Several re-immunization schedules for non-responders have been examined. Doubling the HBV vaccine dose may improve responses, at least in patients with higher CD4 counts and undetectable plasma HIV RNA. The use of adjuvants has not been proven to increase response rates to HBV vaccine.
A special situation is noted in patients positive for antibody to hepatitis B core (anti-HBc) but negative for both HBsAg and anti-HBs. This is infrequent in the general population, but common in HIV individuals [5]. It may reflect clearance of HBsAg but inability to mount an adequate anti-HBs response. Occasionally, an isolated anti-HBc may be a false-positive result, especially in low-risk populations [6]. Or it may reflect ongoing HBV viraemia with mutations in HBsAg. Following HBV booster vaccine, few HIV patients with isolated anti-HBc exhibit an anamnestic response [7]. Therefore, the presence of isolated anti-HBc in HIV patients should not be interpreted as a surrogate marker of protection against HBV. Accordingly, these patients should be tested for HBV DNA. The patient should be treated as for chronic HBV if viraemic for HBV. If HBV DNA is not detectable, the patient should be vaccinated.
Hepatitis B and HIV
Epidemiology
Worldwide, there are an estimated 4 million individuals coinfected with HIV and HBV. Rates of coinfection with HBV vary throughout the world depending on the relative timing of exposure to both viruses [8]. In the USA and Western Europe, the rates of coinfection vary from 6 to 10%. Twenty per cent of HIV patients who are exposed to HBV as adults develop chronic HBV, compared to less than 5% of immunocompetent adults [9]. However, this is different in areas where HBV is endemic with a high rate of vertical and perinatal transmission (Asia and sub-Saharan Africa). Individuals usually develop chronic HBV infection as infants and then acquire HIV as adults. In endemic areas, the prevalence of HBV in HIV patients varies widely from 10 to 25%, depending on the country studied [8]. In the USA and Europe, genotype A is most common in HIV patients [10]. In endemic areas, the HBV genotype of the area predominates in HIV–HBV coinfected patients.
Diagnosis
All HIV positive patients should be tested for HBV (Table 22.1). If negative for all markers, the patient should be vaccinated. HIV patients with positive HBsAg require lifelong ongoing monitoring. If highly active antiretroviral therapy (HAART) is initiated, anti-HBV therapy is mandated (see below). Patients with evidence of past infection (anti-HBs and anti-HBc) or atypical serology occasionally reactivate on HAART [8]. For this reason, HBV should be considered in the differential diagnosis of abnormal aminotransferases even if atypical serology is noted.
Pathogenesis
Hepatitis B is an immune-mediated infection. When HIV is uncontrolled, there is little inflammation in the liver. However, liver damage can occur after immune reconstitution with HAART. For this reason, physicians must know the status of HBV infection before initiating HAART and monitor the HBV status during HAART therapy. Patients with HIV and HBV coinfection have higher HBV DNA levels and lower serum alanine aminotransferase (ALT) levels than those with HBV alone. Liver fibrosis tend to be more advanced and the risk of end-stage liver disease is markedly increased in patients who are coinfected with HIV and HBV [11]. This risk is more pronounced in those with low CD4 counts or alcohol consumption. Despite the availability of HAART, including agents with potent anti-HBV activity, overall mortality and liver-related mortality is increased in HIV–HBV coinfected patients compared to HIV-monoinfected individuals [12]. Patients with elevated serum HBV DNA have lower CD4 counts at HAART initiation. They also have impaired CD4 restoration in response to HAART [13].
Treatment
Treatment endpoints for HBV in HIV patients remain the same as in those without HIV. However, loss of HBeAg or HBsAg as well as seroconversion to anti-HBe and anti-HBs is uncommon. The clinician must first decide whether the patient needs treatment for HIV alone, for HBV alone or for both viruses. If either HIV or both infections meet criteria for therapy, then treatment must include agents active against both viruses. Several nucleoside analogues have activity against both HIV and HBV so they cannot be used without HAART (Table 22.2). Recent guidelines from the USA and Europe recommend the use of two anti-HBV drugs as part of HAART in coinfected persons [14,15]. This is because therapy for HBV in HIV patients is usually lifelong, resistance to nucleoside analogues is common and flares with immune reconstitution can be life-threatening. The use of combination HBV therapy aims to decrease the development of resistance, even though there are limited data on combination therapy in either mono- or coinfected patients. Many HIV patients are lamivudine experienced. Development of lamivudine resistance to HBV is seen in 90% of patients at 4 years [16]. Entecavir has anti-HIV activity and should not be used without HAART [17]. Entecavir may be associated with high rate of resistance if the HBV already has the YMDD mutation in its polymerase gene. Telbivudine may also have activity against HIV [18]. Combination therapy with tenofovir and emtricitabine (or lamivudine) is the preferred treatment at present for coinfected patients. HAART interruption may lead to a flare of HBV, which may be life threatening. For this reason patients should be warned against stopping drugs unless there is careful consultation and monitoring [19].
Table 22.2. HIV and HBV activity of current HBV medications
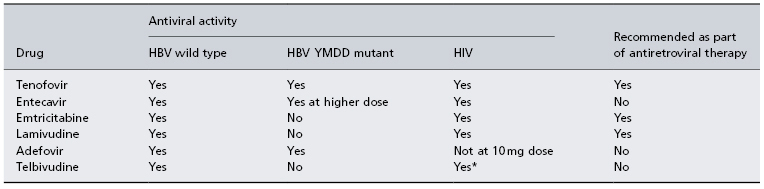
* Limited data as yet.
Treatment options are more limited if the patient does not meet criteria for HIV therapy. European guidelines recommend pegylated interferon (PEG-IFN) or adefovir [14]. PEG-IFN-α has not been well studied in the setting of HIV. It is most effective in immunocompetent patients with low viral load and high ALT, both rare in HBV–HIV coinfected patients. Patients with lower CD4 levels have poorer responses to interferon therapy. US guidelines recommend the early initiation of HAART in these patients because treatment options are limited and HBV disease progresses more rapidly in HIV patients [1,15].
There are multiple causes of abnormal ALT in patients with HIV and HBV (Table 22.3). Cell-mediated immunity plays a central role in the pathogenesis of chronic hepatitis B [20]. In an HIV–HBV coinfected patient with advanced immunosuppression, HBV replication is high but there is little liver inflammation. However, when HAART is initiated, improved cellular immunity can lead to recognition of virally infected hepatocytes and a flare in liver enzymes. Thus reactivation of HBV and abnormal ALT may occur with improved immune response. If due to immune reconstitution, it often resolves with continued therapy. If HAART is initiated without anti-HBV agents then immune reconstitution usually occurs within 6–12 weeks and may be severe. If HAART is stopped, flares in HBV disease often occur. Flares in ALT may be due to the development of drug resistance (e.g. lamivudine) [8]. Abnormal ALT may be due to spontaneous HBV clearance. This occurs rarely in HIV patients. Finally, abnormal ALT may be due to drug toxicity or the development of other liver disease. Liver enzyme flares in HIV–HBV-coinfected patients on HAART therapy need to be carefully interpreted, with concomitant evaluation of serum HBV DNA, in order to correctly assign causality.
Table 22.3. Causes of abnormal liver function tests in HIV patients
In all HIV patients Acute hepatitis A, B or CAcute hepatitis D if patient is HBsAg positive Acute hepatitis B and D coinfection Chronic hepatitis B or C Chronic hepatitis B and D coinfection Non-alcoholic fatty liver disease (NAFLD) Alcoholic liver disease Liver infections (e.g. tuberculosis, MAI) Hepatotoxicity from drugs HAART Anti-infectives Non-steroidal anti-inflammatory drugs Neuropsychiatric medication Over the counter medications, herbals, illicit drugs Liver malignancy In HIV–HBV coinfected patients Reactivation of HBV with improved immune responseImmune reconstitution—continue therapy HAART initiation without anti-HBV therapy—add HBV therapy HAART stopped so patient not receiving HBV therapy—reinstitute HAART therapy Development of HBV drug resistance Spontaneous HBV clearance—occurs rarely in HIV patients Other liver diseases |
HAART, highly active antiretroviral therapy; MAI, Mycobacterium avium complex.
Hepatitis C and HIV
Epidemiology
Of the 35 million people living with HIV worldwide around 20% (approximately 7 million) have chronic hepatitis C. Risk factors for acquisition of both viruses are shared and include intravenous drug use and receipt of contaminated blood (e.g. haemophilia). As with HBV, patients with HIV infection and chronic hepatitis C have faster rates of liver fibrosis progression [21,22]. This is especially seen in those with low CD4 counts. Early introduction of HAART in these patients is encouraged.
Outbreaks of Acute Hepatitis C in HIV Patients
Outbreaks of hepatitis C among homosexual men have been reported in several large European and North American cities since year 2000 [23]. HCV is not efficiently transmitted by sexual contact, unlike HBV and HIV. High levels of sexual promiscuity, certain traumatic sex practices and concomitant ulcerative sexually transmitted diseases (e.g. syphilis), have all been associated with these HCV outbreaks. The increased level of HCV viraemia characteristically seen in HIV persons might further contribute to this enhanced infectivity [24]. HIV-infected patients progress to chronic HCV more frequently than HIV-negative individuals [25]. Response rates to interferon therapy are higher in acute HCV than for chronic HCV. Treatment should not be instituted before 12 weeks of estimated exposure, to allow for spontaneous HCV clearance to occur. A sustained virological response (SVR) to treatment in acute hepatitis C is lower in HIV patients compared to HIV-negative patients (60 versus 80%, respectively). It is unclear whether adding ribavirin to PEG-IFN offers any advantage when treating acute hepatitis C in HIV individuals. However, given the worse prognosis of HCV infection in HIV persons, many experts use both drugs for 24 weeks regardless of HCV genotype [26].
Pathogenesis
Progression to end-stage liver disease occurs more rapidly in this population [27]. The tolerance of antiretroviral agents is much poorer in the presence of underlying chronic hepatitis C, with higher rates of hepatotoxicity [28]. Successful treatment of chronic hepatitis C with clearance of HCV has been associated with a regression of liver fibrosis [29] and with a reduced risk of antiretroviral-related hepatotoxicity [30]. Liver fibrosis can be assessed by liver biopsy, serum markers or elastography. The non-invasive tools are generally accurate in discriminating lack of fibrosis and advanced fibrosis, and less precise with intermediate fibrosis stages [31]. Their predictive value is particularly good for advanced hepatic fibrosis and cirrhosis. However, serum fibrosis markers are generally less reliable in coinfected patients, given the inflammatory nature of HIV disease and/or the frequent use of drugs which may interfere with some fibrosis markers in the blood. This is the case for bilirubin elevations due to atazanavir, γ-glutamyl transpeptidase abnormalities with non-nucleoside reverse transcriptase inhibitors or cholesterol elevations using most ritonavir-boosted protease inhibitors. Patients with repeatedly normal serum ALT may benefit from HCV therapy. Serum ALT is not a good predictor of inflammation in HIV patients. Significant liver fibrosis has been reported in up to 25–40% of coinfected patients with normal ALT and even ‘silent’ cirrhosis in nearly 15% [32].
Treatment of HCV
All HIV persons with chronic HCV infection should be considered potential candidates for HCV therapy. The timing for HCV treatment should be decided on an individual basis. Severe neuropsychiatric disorders, alcohol and drug abuse are generally contraindication to HCV treatment. Host factors, including liver fibrosis stage, CD4 counts and patient motivation, are the most important variables that determine who will respond to HCV therapy (Table 22.4) [27]. HIV–HCV patients with liver decompensation (ascites, gastrointestinal bleeding, hepatic encephalopathy, etc.) should not be treated with PEG-IFN, given the higher risk of liver decompensation and serious side effects. These patients should be evaluated for liver transplantation. However, patients with compensated cirrhosis (Child–Pugh class A) may be treated with PEG-IFN plus ribavirin. These patients will benefit most from HCV clearance and potential reversal of severe liver fibrosis. Other predictors of response include estimated length of HCV infection, the severity of liver disease, the extent of HIV suppression, HCV genotype and viral load (Table 22.4). Genetic polymorphisms in the IL28B gene influence treatment response in HCV monoinfected patients [33]. It has not been studied in coinfected patients yet. Treatment responses are inversely related to pretreatment baseline CD4 [14]. Insulin resistance is a negative predictor of response and is prevalent in coinfected patients, at least in part due to antiretroviral therapy including ritonavir-boosted protease inhibitors. Treatment compliance is critical. Adequate selection of treatment candidates, psychological and/or psychiatric support, and use of growth factors to avoid dose reductions of either PEG-IFN and/or ribavirin is required.
Table 22.4. Factors associated with sustained virological response to HCV therapy in HIV patients
Host Genetics (white ethnicity, IL28b polymorphisms)Younger age Minimal liver fibrosis Low body mass index No insulin resistance No hepatic steatosis Higher CD4 count No polysubstance abuse No psychiatric disease Virus Genotypes 2/3Low baseline HCV RNA Undetectable week 4 HCV RNA Treatment Adequate pegylated interferon doseWeight-based ribavirin dose Good adherence No concurrent didanosine, zidovudine or abacavir Haematopoietic growth factors as needed |
Toxicity of PEG-IFN and/or ribavirin and poorer response rates are more frequent in severely immune deficient patients. Interferon generally causes a decline in the absolute CD4 count which reverses after the end of treatment [34]. In drug-naïve coinfected patients with low CD4 counts, antiretroviral therapy should be initiated first. Once CD4 cells have improved and plasma HIV RNA is controlled, HCV therapy should be reassessed. Conversely, in antiretroviral-naïve individuals with good CD4 counts, hepatitis C should be treated first. These patients will further benefit from improved tolerance of antiretroviral drugs [30]. In patients with CD4 counts below 200 cells/mm3 already on HAART, HCV treatment is usually deferred unless other favourable treatment predictors are present (e.g. HCV genotypes 2 or 3, low HCV load).
HCV kinetics is important in HIV patients. Virological responses can be assessed at early time points to identify who will and who will not respond to therapy. The best positive predictor of SVR is a negative HCV RNA after 4 weeks of therapy (RVR, rapid virological response). The best negative predictor is a less than 2 log decrease in HCV RNA after 12 weeks of therapy (EVR, early virological response) [35]. HIV patients have higher baseline HCV RNA levels and are less likely to have RVR and SVR [36].
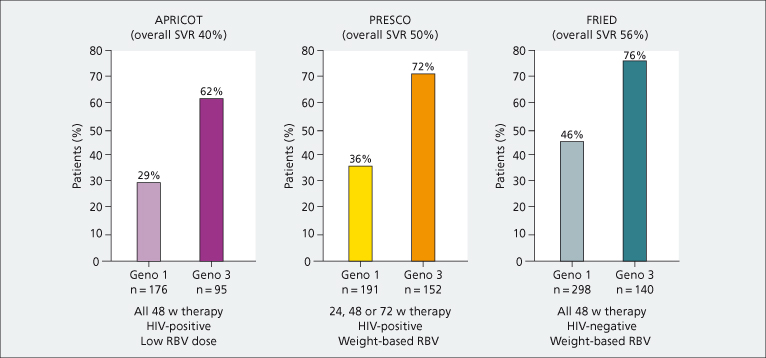
Optimal PEG-IFN and ribavirin dosing and duration are currently under development. Adequate exposure to ribavirin is crucial to maximize responses to HCV therapy, particularly in HIV-coinfected patients. Weight-based ribavirin dosing is recommended [37]. Pharmacokinetic studies have shown a good correlation between ribavirin plasma levels and HCV RNA responses. This is rarely practical. Figure 22.2 shows the proportion of patients achieving SVR in pivotal trials as a function of distinct ribavirin doses and HIV status. There is no proven efficacy of higher doses of PEG-IFN in coinfected patients.
Fig. 22.2. Proportion of patients with sustained virological responses (SVR) in three large trials (APRICOT, PRESCO, FRIED) in HIV-positive and HIV-negative patients using low or weight-based ribavirin (RBV) doses (intent-to-treat analysis).
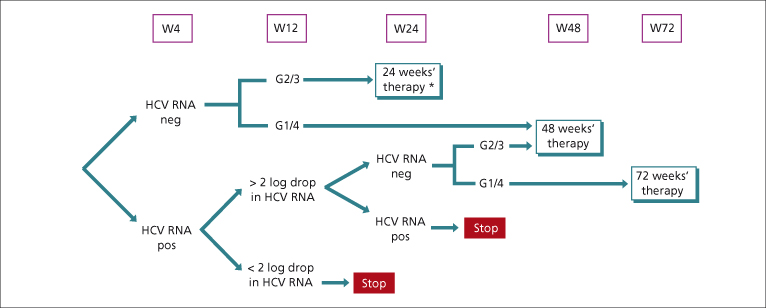
Guidelines recommended that duration of treatment in coinfected patients should be of 48 weeks regardless of HCV genotype (Fig. 22.3) [26,38]. Studies conducted in HCV-monoinfected patients have shown that RVR in patients treated with PEG-IFN–ribavirin is the best predictor of SVR. Attainment of RVR may permit a shorter duration of therapy. HIV–HCV coinfected patients with RVR also appear to have a high chance of SVR [39]. RVR is uncommon as HCV load is higher in HIV patients. But if HIV–HCV patients achieve RVR they may achieve SVR with a shorter duration of treatment [26]. Shorter periods of therapy (24 weeks) can be used in HIV patients with all of the following: (1) HCV genotype 2 and 3 with (2) RVR, (3) HCV load is low, (4) good adherence, (5) mild to moderate liver fibrosis and (6) weight-based ribavirin dosing [14,26]. For the remainder of HCV genotypes 2 and 3 patients, 48 weeks of therapy is recommended. In patients with HCV genotype 1 and 4, extension of treatment beyond 48 weeks is recommended in those with EVR but no RVR, as long as the medication is well tolerated. However, high drop-out rates limit the benefit of this strategy.
Fig. 22.3. Proposed optimal duration of HCV therapy in HCV–HIV-coinfected patients [14,26]. * Only in patients with baseline low viral load and minimal liver fibrosis.
Antiretroviral Drugs During HCV Therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








