Bile Pigments
Bilirubin
Bilirubin metabolism is described in detail in Chapter 11.
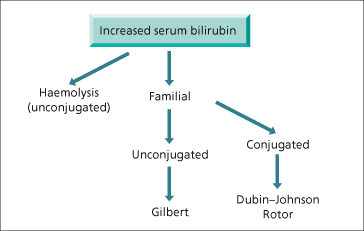
Total bilirubin is increased in cholestatic and hepatocellular liver disease more commonly than infiltrative disease. It is often associated with a rise in liver enzymes. Bilirubin is predominantly conjugated and water soluble. Patients with marked hyperbilirubinaemia (bilirubin > 425 µmol/L) often have severe liver disease coexisiting with renal dysfunction or another cause of unconjugated hyperbilirubinaemia, such as haemolysis [3]. An isolated rise in bilirubin without enzyme elevation should first be fractionated to determine if the aetiology is familial or due to haemolysis (Fig. 2.2).
Serum bilirubin estimations are based on the van den Bergh diazo reaction, which involves the spectrophotometric detection of azo derivatives derived by the reaction of plasma with the diazonium ion of sulphanilic acid [4]. This reaction separates bilirubin into a water-soluble direct form representing conjugated bilirubin and an indirect, lipid-soluble form representing unconjugated bilirubin. These diazo reactions are subject to error, particularly at low total serum bilirubin concentrations. More accurate methods for estimation include alkaline methanolysis with chloroform extraction, high performance gas liquid chromatography (HPGLC), thin layer chromatography (TLC) and spectrophotometric determination, but are too elaborate to be clinically useful [5].
Faecal inspections are an important investigation in jaundice. Clay-coloured stools indicate cholestatic jaundice but may also occur in hepatocellular jaundice. The colour will be normal in haemolytic jaundice. Rarely, pale stools occur in very severe bilirubin glucuronyl transferase deficiency.
Bilirubin cannot be detected in the urine of normal subjects as bilirubin is predominantly unconjugated, insoluble in water and bound to albumin. In contrast, bilirubin glucuronides, the products of bilirubin conjugation, are water soluble. They appear in the urine even when serum total bilirubin is normal as the renal threshold for glomerular filtration of conjugated bilirubin is low. Conjugated bilirubin, however, will bind covalently to albumin when jaundice is prolonged and severe, giving rise to a complex called δ bilirubin (or bilioprotein) [6]. δ bilirubin has a long half-life, cannot be renally cleared and accounts for the absence of bilirubinuria and slow resolution of jaundice in patients recovering from severe hepatobiliary disease.
Urobilinogen
Bacterial β-glucuronidases convert bilirubin in the colon to a series of colourless tetrapyrroles collectively called urobilinogen of which 80–90% is normally excreted in the faeces either unchanged or as oxidized orange derivatives called urobilins. The remaining 10–20% is absorbed and undergoes an enteric circulation with re-excretion into bile by the liver while a small proportion is excreted in the urine. This complex process depends on several factors such as urine flow rate and pH. Spot urinary urobilinogen is a poor predictor of hepatic disease with a high proportion of false-negative results [7].
Bromsulphalein
The intravenous dye bromsulphalein (BSP) is rapidly removed by the liver with a first-pass clearance between 50 and 80%. Its removal is related to hepatic blood flow and is excreted into the biliary canaliculus by an ATP-dependent export pump (MRP2, ABCC2), a member of the ATP-binding cassette protein family [8]. This test is rarely performed now due to the cost, occasional side-effects such as anaphylaxis, lack of specificity and inconvenience. However, it does have a role in the diagnosis of Dubin–Johnson syndrome and its differentiation from Rotor’s syndrome [9]. A blood sample is taken 45 min and 2 h after injection. A higher level of BSP at 2 h rather than at 45 min is diagnostic of Dubin–Johnson syndrome and reflects release of conjugated BSP into the blood stream after normal initial uptake. In Rotor’s syndrome, BSP clearance is slow with no secondary rise.
Serum Enzyme Tests
These tests usually indicate the type of liver injury, whether hepatocellular, cholestatic or infiltrative but cannot differentiate one form of hepatitis from another or determine whether cholestasis is intra- or extrahepatic. They are valuable in directing specific serological tests, imaging or liver biopsy to reach the diagnosis. Only a few tests are necessary and the combination of AST, ALT, ALP and bilirubin is usually adequate. Ideally, normal ALT values should be adjusted for body mass index and sex [10]. During pregnancy, ALT, AST and γ-GT levels, as well as bile acid and bilirubin concentrations remain within the normal range, whereas an elevated alkaline phosphatase is of placental origin during the third trimester [11].
Aminotransferases
The aminotransferases (previously called aminotransaminases) catalyse transfer of amino groups from either aspartate or alanine to the keto group of α-ketoglutaric acid forming oxaloacetic acid (OAA) and pyruvic acid, respectively. These enzymes are important in gluconeogenesis as they catalyse glucose synthesis from non-carbohydrate sources. Enzymatic reduction of oxaloacetic acid and pyruvic acid to malate and lactate, respectively, is coupled with oxidation of the reduced form of nicotinamide dinucleotide (NADH) to nicotinamide dinucleotide (NAD). As only NADH absorbs light at 340 nm, this reaction can be followed spectrophotometrically to accurately assay these enzymes.
Aspartate aminotransferase (AST; serum glutamic oxaloacetic transaminase or SGOT) is an isoenzyme located in the cytoplasm and mitochondria of many tissues. Although normal AST serum activity is cytosolic in origin, 80% of AST activity within the liver is mitochondrial and predominates in periportal hepatocytes. In decreasing order of concentration AST is present in large quantities in liver, heart, skeletal muscle, kidneys, brain, pancreas, lungs, leucocytes and erythrocytes. Macro-AST is a rare condition characterized by isolated AST elevation due to binding of AST with an immunoglobulin which is not cleared by the blood or kidneys [12]. It is a benign condition and is not reflective of liver disease. Markedly low AST levels have been reported in patients on chronic haemodialysis, possibly due to dialysis or pyridoxine deficiency [13,14].
Alanine aminotransferase (ALT; serum glutamic pyruvic transaminase or SGPT) is a cytosolic enzyme also present in liver. Although the absolute amount is less than AST, a greater proportion is present in liver compared with kidney, heart and skeletal muscles. A serum increase is therefore more specific for liver damage than AST.
Transferase determinations with viral serologies are useful in the early diagnosis of viral hepatitis, but there is no correlation between transferase level with either the degree of hepatocyte necrosis or prognosis. Measurements should be performed promptly as these enzymes have short half-lives (AST 12–22 h; ALT 37–47 h) [15]. Patients may develop fatal acute hepatic necrosis despite falling transaminase values.
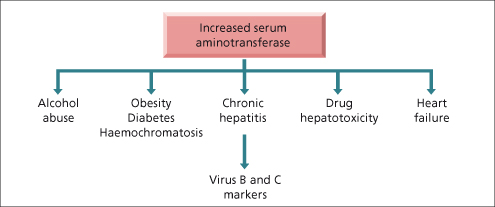
Routine screening may show unexpectedly raised aminotransferase levels (Fig. 2.3). These are often due to non-alcoholic fatty liver disease (NAFLD), alcohol abuse, viral hepatitis and haemochromatosis. Less common causes include autoimmune hepatitis, α1-antitrypsin deficiency, Wilson’s disease, drug-induced liver disease and non-hepatic disorders such as Addison’s disease [16], anorexia nervosa [17], coeliac disease [18] and hyperthyroidism [19]. Important causes of markedly elevated transaminases are viral hepatitis (including herpes simplex hepatitis), paracetamol (acetaminophen) or other drug-induced hepatotoxicity, ischaemic hepatitis and severe autoimmune hepatitis [20]. Calculous biliary obstruction with cholangitis is an important but frequently under appreciated cause of AST elevation greater than 10 times the upper limit of normal, which may improve with antibiotics over 48–72 h despite unresolved obstruction.
Fig. 2.3. Algorithm for managing a patient with an isolated increase in serum aminotransferase on routine screening.
Very high levels are unusual in ALD and suggest a coexisting disorder such as paracetamol toxicity or acute viral hepatitis. A ratio of AST to ALT greater than two may be useful in diagnosing ALD. This occurs because damage is primarily mitochondrial (thus more AST is released systemically) and ALT synthesis is more sensitive than AST to pyridoxal 5-phosphate deficiency, leading to lower serum ALT levels [21]. An elevated AST to ALT ratio has also been described as a specific but non-sensitive marker of advanced fibrosis or cirrhosis in NAFLD [22] and chronic hepatitis C [23].
Alkaline Phosphatase
The alkaline phosphatases (ALP) are a group of enzymes that catalyse hydrolysis of phosphate esters at neutral pH. Magnesium and zinc are important co-factors. ALP in the liver is cytosolic, associated with sinusoidal and canalicular membranes and rises in cholestasis and to a lesser extent when liver cells are damaged (Fig. 2.4). ALP is present, in decreasing order of quantity, in placenta, ileal mucosa, kidney, bone and liver but more than 80% of serum ALP is from the liver or bone. ALP half-life is 3 days. Bone, liver and kidney ALP are coded by the same gene and share a common protein structure but differ in their carbohydrate content [24]. Mechanisms of the increase are believed to be related to increased hepatobiliary synthesis from enhanced translation of messenger ribonucleic acid of ALP and serum secretion through canalicular leakage into the sinusoid rather than failure to excrete ALP. Due to de novo ALP synthesis in acute biliary obstruction, serum levels are initially normal in contrast to marked transferase elevations. Serum hepatic ALP may be distinguished from bone ALP by isoenzyme fractionation but this is not routinely carried out as a concomitant rise in γ-GT confirms a hepatobiliary source.
Fig. 2.4. Algorithm for managing a patient with an isolated increase in serum alkaline phosphatase or serum γ-glutamyl transpeptidase (γ-GT). CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; PBC, primary biliary cirrhosis.
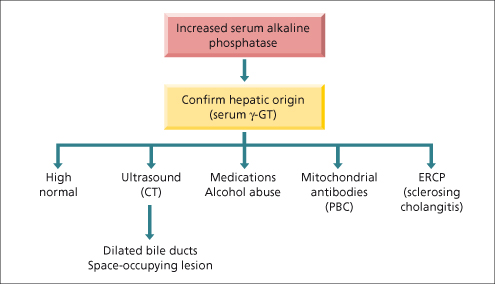
An isolated rise in ALP may also be of intestinal origin, as observed in patients with blood groups O and B who secrete intestinal ALP postprandially. As these enzymes may remain elevated for up to 12 h, levels must be determined under fasting conditions [25]. Up to 52% of patients with mild isolated ALP elevations (less than twofold elevation) will have enzyme normalization within 1–3 months although in hospitalized patients, sepsis in the absence of jaundice may account for up to 32% of cases [26].
Raised ALP levels are sometimes observed with primary or secondary hepatic tumours, even without jaundice or involvement of bone. Increased values without jaundice are also found with other space-occupying lesions or infiltrative disease such as amyloid, abscess, lymphoma or granulomas. Non-specific mild elevations are seen in a variety of conditions including Hodgkin’s disease, heart failure, hyperthyroidism and up to 15% of patients with renal cell carcinoma in the absence of involvement of the hepatobiliary system or bone (Stauffer’s syndrome). Low ALP levels are associated with hypothyroidism, Wilson’s disease with haemolysis, congenital hypophosphatasia, pernicious anaemia, zinc deficiency, severe hepatic insufficiency and in children recovering from severe enteritis.
Gamma Glutamyl Transpeptidase or Transferase
Gamma glutamyl transpeptidase (γ-GT) is a membrane-bound enzyme that catalyses transfer of γ glutamyl groups of peptides such as glutathione to other amino acids. Levels are increased in cholestasis and hepatocellular disease and occur in the same spectrum of hepatobiliary diseases as elevated ALP. γ-GT is ubiquitous but in decreasing order of abundance is present in proximal renal tubule, liver, pancreas (acinar cells and ductules) and intestine. Serum γ -GT activity arises primarily from the liver and, within the hepatobiliary system, is present in highest concentration in the epithelium lining of fine biliary ducts. The main role of this test is to confirm a raised ALP is of hepatobiliary origin.
An isolated rise in γ-GT is seen in patients with alcohol abuse, even without liver disease, due to microsomal enzyme induction and impaired clearance (half-life of 7–10 days increases to 28 days). Screening for γ-GT may have led to more alcohol abusers being identified although levels do not rise in one-third of individuals. There also is no correlation between alcohol consumption and elevated serum γ-GT levels with hepatic γ-GT in patients with biopsy-proven alcoholic liver disease. An increased level can lead to over-investigation in an individual who has never taken alcohol or a social drinker who has never abused alcohol.
Unfortunately, many factors influence the level so that increases are non-disease-specific. Disorders include hepatobiliary disease, alcoholism, chronic obstructive airways disease, diabetes mellitus, hyperthyroidism, rheumatoid arthritis and several medications such as barbiturates, carbamazepine, cimetidine, furosemide, heparin, isotretinoin, methotrexate, oral contraceptives, phenytoin and valproate. On the other hand, it has excellent sensitivity and a high predictive value for screening for biliary tract disease such that levels are rarely normal in intrahepatic cholestasis. Exceptions are subtypes of progressive familial intrahepatic cholestasis (type 1 or Byler’s disease and type 2) and benign recurrent intrahepatic cholestasis type 1 (or Summerskill’s syndrome) [27].
Lactic Dehydrogenase
Lactic dehydrogenase (LDH) is a cytoplasmic enzyme with five isoenzymes present in serum. Marked increases are found in patients with neoplasms and hepatic involvement and ischaemic hepatitis. The ALT : LDH ratio has been reported to discriminate between acute viral hepatitis (greater than 1.5) from ischaemic hepatitis and paracetamol toxicity (less than 1.5), with excellent sensitivity and specificity [28].
Quantitative Assessment of Hepatic Function (Table 2.2)
Chronic liver diseases pass through a long period of minimum non-specific symptoms (‘compensated’) until the final stage of ascites, jaundice, variceal bleeding, encephalopathy and precoma (‘decompensated’). Serum albumin and prothrombin time give some indication of the synthetic function of the liver, but this is usually maintained until late disease. Serial estimates of quantitative liver function in the early stages may be helpful for monitoring treatment and assessing prognosis but have no diagnostic role. Such tests suffer from the drawback of their complexity (multiple blood samples or measurement of isotopes). The lack of a major impact above routine laboratory tests and CP score or MELD is reflected in their present role in clinical research rather than the routine management of patients. These investigations have been reviewed elsewhere [29] and will not be discussed.
Lipid and Lipoprotein Metabolism
Lipids
The liver is central to lipid (cholesterol, phospholipid, triglyceride) and lipoprotein metabolism. Lipoproteins, hydrophobic within and hydrophilic on the outside, allow lipid transport in the plasma.
Cholesterol (from the Greek words chole, bile, and stereos, solid) is found in cell membranes and is a precursor of bile acids and steroid hormones. It is synthesized in all tissues, but the most active site of synthesis is the liver. Cholesterol derived from intestinal absorption reaches the liver in chylomicron remnants. Cholesterol synthesis takes place mainly from acetyl coenzyme A (CoA) in the microsomal fraction and in cytosol (Fig. 2.5). The rate-limiting step of cholesterol synthesis is the conversion of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) to mevalonate by the enzyme HMG-CoA reductase which is located almost exclusively in periportal cells. Synthesis is increased in biliary duct obstruction, terminal ileal resection, biliary or intestinal lymph fistula and medications such as cholestyramine, corticosteroids and thyroid hormones. Cholesterol synthesis is inhibited by bile acids, cholesterol feeding, fasting and medications such as clofibrate, nicotinic acid and statins.
Fig. 2.5. Hepatic cholesterol balance. Free cholesterol is derived from intracellular synthesis, and from the uptake of chylomicron remnants and lipoproteins from the circulation. Storage is as cholesterol ester: ACAT (acyl CoA-cholesterol ester transferase, which esterifies free cholesterol to fatty acids) and CEH (cholesteryl ester hydrolase, which hydrolyses the ester linkage). Bile acids are synthesized from free cholesterol, and both are secreted into bile. 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase is the rate-limiting step. HDL, high density lipoprotein; LDL, low density lipoprotein.
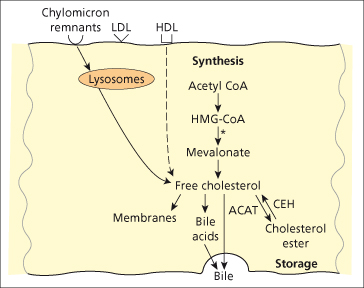
Cholesterol in membranes and in bile is present almost exclusively as free cholesterol. Bile provides the only significant route for cholesterol excretion. In plasma and tissues such as liver, adrenal and skin, cholesterol esters are also found, which are more non-polar and metabolically inactive than free cholesterol. Esterification is carried out by plasma lecithin cholesterolacyl transferase (LCAT) [30].
Phospholipids contain one or more phosphoric acid groups and another polar group such as a heterogeneous base, for example choline or ethanolamine with long-chain fatty acid residues. Phospolipids are important constituents of cell membranes and participate in many reactions. The most abundant phospholipid in plasma and most cellular membranes is phosphatidyl choline (lecithin) which accounts for 66% of all phospholipids. Phosphatidyl secretion into bile (22%) is also greater than cholesterol secretion (4%), and is promoted by bile salts secretion into the canalicular lumen via canalicular bile acid transporter. This induces vesiculation of the outer, canalicular membrane leading to increased supply of phospholipids to the inner, cytoplasmic membrane by phosphatidylcholine transfer protein and sterol carrier protein 2 [31]. Phospholipid translocation from inner to outer membrane occurs via the multidrug resistance protein 2 (MDR2) pathway which combine with bile salts to form vesicles [32].
Triglycerides are simpler compounds than the phospholipids. They have a backbone of glycerol, the hydroxy groups of which have been esterified with fatty acids. Naturally occurring triglycerides contain a variety of fatty acids; they act as a store of energy and also a method of transport of energy from the small intestine and liver to peripheral tissues.
Lipoproteins
These are essential for the circulation and metabolism of lipids. They are separated by their differing density on ultracentrifugation, which explains their nomenclature. Their surface comprises apolipoprotein, of several different types (Table 2.3), free cholesterol and phospholipids. Inside there is cholesterol ester, triglycerides and fat-soluble vitamins. There are two prominent metabolic cycles for lipoproteins: one is involved in fat absorbed from the intestine, and the other is responsible for the handling of endogenously synthesized lipid (Fig. 2.6).
Table 2.3. Properties of lipoproteins
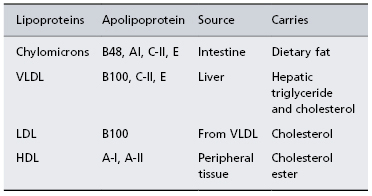
HDL, high density lipoprotein; LDL, low density lipoprotein; VLDL, very low density lipoprotein.
Fig. 2.6. The role of the liver in lipoprotein metabolism. CH, cholesterol; FFA, free fatty acid; LDLR, LDL receptor; LL, lipoprotein lipase; TG, triglyceride. (For lipoproteins see Table 2.3.)
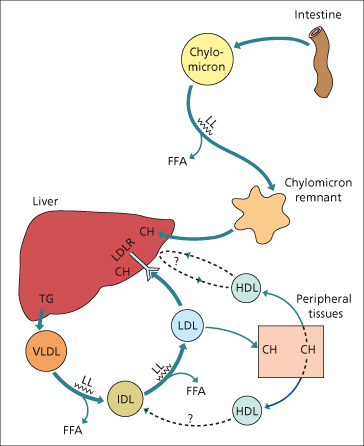
Dietary fat is absorbed from the small intestine and incorporated into chylomicrons [33,34]. These enter the circulation via the thoracic duct where triglyceride is removed by lipoprotein lipases and utilized or stored in tissue. The chylomicron remnant is taken up by the liver by the low density lipoprotein (LDL) receptor-related protein [34]. The cholesterol enters metabolic pathways or plasma membranes, or is excreted in bile.
In the endogenous pathway, cholesterol and triglyceride leave the liver in very low density lipoprotein (VLDL), which is synthesized predominantly in the mitochondria of perivenous hepatocytes. In the circulation, triglyceride is removed by lipoprotein lipase. As a result, VLDL particles become smaller, forming intermediate density lipoprotein (IDL), and then LDL, the major carrier for cholesterol. The predominant route for removal of LDL is by LDL receptors on the liver surface, but receptors on other cells are also important in atheromatous plaques formation. High density lipoprotein (HDL) facilitates cholesterol removal from peripheral tissues. Cholesterol is transported out of the cell by the cholesterol-efflux regulatory protein, expressed from the adenosine triphosphate (ATP) binding cassette transporter 1 gene (ABC1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree