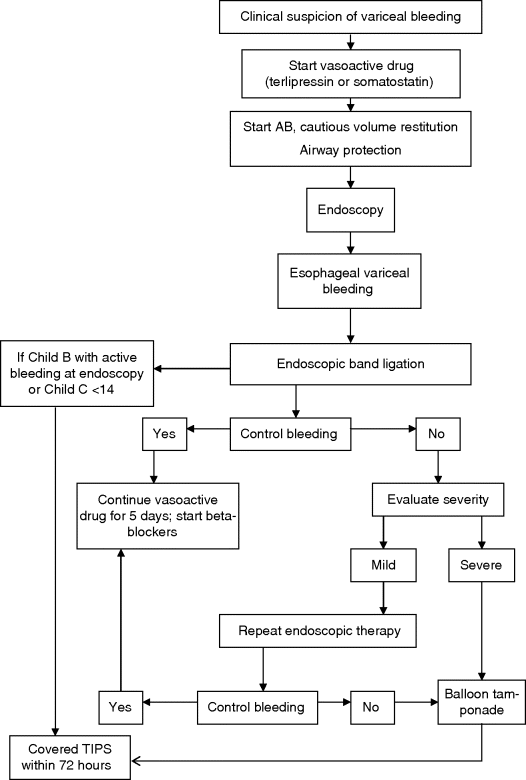
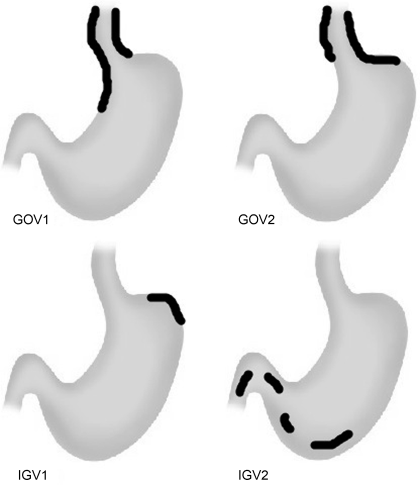
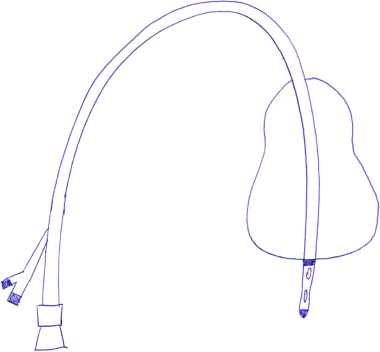
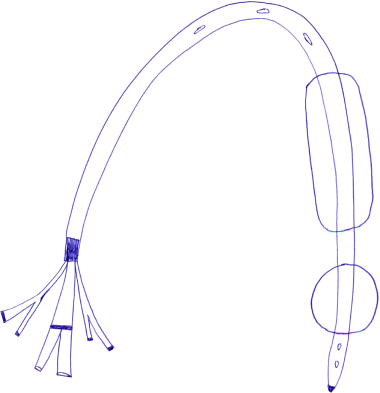
Chapter 13 Isabelle Colle,1 Xavier Verhelst,2 Anja Geerts, 2 and Hans Van Vlierberghe2 1Department of Hepatology and Gastroenterology Algemeen Stedelijk Ziekenhuis (ASZ), Aalst, Belgium and Ghent University 2Department of Hepatology and Gastroenterology Ghent University Hospital Ghent Belgium Acute variceal bleeding is a notorious complication of portal hypertension with a high morbidity and mortality. When the portal pressure (measured by the hepatic venous pressure gradient, HVPG) is ≥10 mmHg, portosystemic collaterals and varices begin to form. These varices subsequently grow in size and the wall becomes thinner with increasing wall tension according to Laplace’s law. Finally, the wall ruptures resulting in a variceal bleed [1]. Acute variceal bleeding occurs in 25–40% of patients with cirrhosis [2] and mortality rates vary from 10% to 50% per episode [3–5]. Up to one-third of survivors will experience rebleeding within 6 weeks [6] and up to 62% at 2 years [1]. Variceal hemorrhage generally occurs in patients diagnosed with end-stage chronic liver disease but it can be the first presentation of an unknown underlying liver cirrhosis. Rarely, it is a consequence of noncirrhotic portal hypertension or portal vein thrombosis. The typical clinical presentation is a patient with liver cirrhosis presenting with hematemesis, coffee-ground emesis, melena, or rectorrhagia. Severity of bleeding and prognosis are associated with several factors. Patients with high portal pressures (HVPG ≥20 mmHg) have worse outcomes. Advanced liver failure is a bad prognostic factor. In Child–Pugh A patients, mortality is close to zero, in Child–Pugh B it is 10%, and in Child–Pugh C patients almost one in three will die. Patients with massive bleeding presenting with hemorrhagic shock and patients with several comorbidities such as hepatocellular carcinoma, portal vein thrombosis, acute alcoholic hepatitis, sepsis, and organ failure have a worse outcome [1]. Endoscopy is the gold standard for diagnosing upper variceal bleeding and should be performed within 12 hours [7,8]. Before endoscopy can be performed, a clinical approach is essential to establish a tentative diagnosis. The following questions should be answered during this initial evaluation [7]. The first goals in the management of acute variceal bleeding are to control the bleeding, prevent early rebleeding, and treat concomitant complications, thus allowing the patient to survive the 5-day acute bleeding period [1]. Acute variceal bleeding should be managed by a multidisciplinary team involving experienced medical intensive care staff, well-trained nurses, clinical hepatologists, endoscopists, interventional radiologists, and surgeons [10]. An algorithm is proposed in Figure 13.1. Figure 13.1 Algorithm for esophageal variceal bleeding based on the Baveno V guidelines. AB, antibiotic; EBL, endoscopic band ligation; TIPS, transjugular intrahepatic portosystemic shunt. The management of a patient with acute variceal bleeding includes resuscitation, vasoactive medication, and endoscopy. Resuscitation is guided by the classic mnemonic ABC (Airway, Breathing, Circulation). Any doubt about airway safety should lead to prompt endotracheal intubation [11]. Fluid resuscitation should be initiated as soon as possible in patients with signs of shock (tachycardia >100/minute, systolic blood pressure <100 mmHg, or increased lactate levels) with a target of mean arterial pressure (MAP) >65 mmHg [12]. Albumin 5% is the preferred volume expander. After reaching hemodynamic stability, volume replacement at 30 mL/kg/24 hours can be performed in the absence of cardiac or renal contraindications. Transfusion of packed red blood cells above 8 g/dL is not appropriate, unless the patient has a history of coronary or cerebrovascular disease [13,14], as blood transfusion can induce a rebound increase in portal pressure, cause rebleeding or sustained bleeding [15]. Vasoactive drugs (terlipressin and somatostatin) aim to cause splanchnic vasoconstriction, thereby decreasing portal pressure and reducing or even stopping bleeding from varices. Bleeding is stopped in 75–80% of patients and the risk of rebleeding is reduced. Therefore, vasoactive medication should be started as early as possible when a variceal bleeding is presumed [16]. Vasoactive medication should be continued for 5 days according to the most recent Baveno V guidelines [8]. Different vasoactive drugs are locally available around the world. The first choice should be terlipressin because it is the only drug that has been proved to bring about a reduction in mortality in acute variceal bleeding [17,18]. Somatostatin and its analogs are second choice considering that they have not demonstrated a reduction in mortality [19]. However, a recent meta-analysis claimed to show no difference in efficacy among octreotide, somatostatin, terlipressin, vasopressin, and vapreotide [20] including in mortality, but there were some methodologic issues. Vasoactive drugs should always be used in combination with endoscopic treatment. Terlipressin is a long-acting triglyl lysine derivative of vasopressin. It is started as an intravenous (IV) bolus (<50 kg = 1 mg; 50–70 kg = 1.5 mg; >70 kg = 2 mg) followed by an IV bolus of 1–2 mg every 4 hours for 48 hours and then 1 mg every 4 hours until day 5. Terlipressin improves the rates of bleeding and survival [21]. Fewer side effects seem to appear when it is given in a continuous IV infusion (6 mg/24 hours) as proposed in hepatorenal syndrome [22]. It is contraindicated in patients with coronary insufficiency, peripheral vascular disease, pregnancy, heart rhythm disease, severe bronchial asthma, uncontrolled arterial hypertension, or epilepsy. If terlipressin cannot be used, somatostatin is a good alternative. Somatostatin treatment starts with a continuous IV infusion of 6 mg over 24 hours followed immediately by a bolus of 250 μg within 5 minutes. The bolus can be repeated up to three times during the first hour if bleeding is uncontrolled. The dosage can be increased to 12 mg over 24 hours in cases of active bleeding. There are no important side effects or contraindications [1,19]. Minor complications are seen in up to 30% of patients and include nausea, vomiting, and hyperglycemia [19,23]. Somatostatin is also used in children; however, large clinical trials are not available. It is started with a continuous IV infusion of 3.5 μg/kg/hour immediately followed by a bolus of 3.5 μg/kg IV over 5 minutes [24]. A second alternative is octreotide. This somatostatin analog has a longer half-life than somatostatin [25]. The safety profile is similar to that of somatostatin. However, clinical trials are limited and could not demonstrate a reduction in mortality [26]. This may be because of the rapid development of tachyphylaxis [25]. Octreotide only showed a reduction in rebleeding after sclerotherapy [27]. There is no evidence for the use of proton pump inhibitors [8] or antifibrinolytics [28] in these patients. The use of prophylactic antibiotics is mandatory in patients with variceal bleeding, as they decrease infectious complications and reduce mortality [29–31]. Patients with variceal bleeding need an active search for infections, including blood, ascites, and urine culture. Empiric antibiotic regimens should be based on local resistance patterns. Proposed first line antibiotics are amoxicillin-clavulanic acid, quinolones, or third line cephalosporins. In patients with advanced cirrhosis, a third line cephalosporin may be advised (e.g., ceftriaxone 1 g/day), especially in inpatients with high quinolone resistance or those chronically taking norfloxacin for prevention of spontaneous bacterial peritonitis [32]. The duration should be limited to 7 days according to the American Association for the Study of Liver Diseases (AASLD) guidelines [30]. Many cirrhotic patients present with coagulation disorders, low platelet counts, and intake of antiplatelet or antivitamin K drugs. However, we should remind that prothrombin time/international normalized ratio is not a reliable indicator of the coagulation status in these patients [8]. For this, the use of coagulation factors, fresh frozen plasma, and platelets is not encouraged, as these could increase portal pressure and maintain the bleeding. However, when the platelet count is lower than 30 000/μL, platelet transfusion can be advised in cases of persistent bleeding. Withdrawal of antiplatelet drugs and antivitamin K medication can cause thrombotic events and should only be stopped after consultation with a cardiologist [7]. Endoscopy should be performed as soon as possible but within 12 hours [8,16,33]. Hemodynamically unstable and high risk patients should be transferred to an intensive care unit to perform endoscopy in a safe environment. Prophylactic endotracheal intubation should be performed in patients with active hematemesis, hemodynamic instability in spite of adequate volume loading, absence of cooperation, or Glasgow Coma Scale (GCS) <8. Stomach cleansing can be performed by administrating 250 mg erythromycin IV over 5 minutes, 20 minutes before endoscopy [34–36]. This may help to improve the endoscopic view and hence lead to a better therapeutic performance. Endoscopic diagnostic criteria for variceal bleeding are active bleeding from a varix, presence of a white nipple (fibrin clot) on a varix, or the presence of varices without other potential source of bleeding and fresh blood in the stomach [1,11,37]. Endoscopic therapy should be performed during the same procedure by a skilled endoscopist. Endoscopic band ligation (EBL) is preferred for esophageal varices as it is superior for bleeding control and rebleeding compared to sclerotherapy and has fewer adverse events. However, sclerotherapy can be a valuable alternative if EBL is not available or cannot be performed for technical reasons [1]. Gastric Varices are Responsible for 5–10% of Variceal Bleeds, But are More Severe and Have a Higher Mortality [38]. Gastric varices can be divided into four types according to the Sarin classification (Figure 13.2) [38]. This classification is based upon their relation with esophageal varices and location. Gastroesophageal varices (GOV) are associated with esophageal varices along the lesser curvature (GOV1) or along the fundus (GOV2). Isolated gastric varices (IGV) appear in the fundus (IGV1) or on other sites in the stomach or the first part of the duodenum (IGV2). If these appear after endoscopic obliteration of esophageal varices, they are classified as secondary gastric varices. Gastric varices are more frequent in individuals with portal hypertension resulting from extrahepatic portal vein thrombosis. Gastric varices located in the fundus have a higher bleeding incidence. Other risk factors for a first hemorrhage are advancing Child–Pugh stage, presence of red spots, and increasing size of the varices [39]. Figure 13.2 Classification of gastric varices according to Sarin et al., 1992 [38]. GOV, gastroesophageal varices; IGV, isolated gastric varices. Reproduced with permission of John Wiley & Sons. The therapeutic approach remains empirical, as large randomized controlled trials (RCTs) are not available. Acute bleeding from gastric varices is challenging. Based on the extrapolation of data from esophageal varices, the use of vasoactive drugs is recommended [40]. GOV1 are the most common type of gastric varices (74%) [38] and can endoscopically be treated like “conventional” esophageal varices with EBL. The other types of gastric varices require a patient-tailored approach but most of them will need injection of glue agents such as N-butyl-2-cyanoacrylate (Histoacryl©) or isobutyl-2-cyanoacrylate (Bucrylate©). This procedure can be technically demanding during active bleeding. It should be performed by a skilled and experienced endoscopist. Major complications can occur because obturation can extend beyond the varices and pulmonary and cerebrovascular embolization has been reported [40]. A small series reports successful treatment of gastric varices by thrombin injection [41]; however, these findings have not been confirmed in larger trials. Failure to control bleeding was defined by the Baveno V guidelines as a fresh hematemesis ≥2 hours after the start of a drug treatment, or therapeutic endoscopy; a 3 g/dL drop in hemoglobin if no transfusion is administered; or death. This should lead to a change of treatment options. Balloon tamponade is indicated for uncontrolled bleeding as a bridge to a definitive therapy within 24 hours. Two types are available: a Linton tamponade (Figure 13.3) can treat esophageal and gastric varices, whereas a Sengstaken–Blakemore tamponade (containing two balloons) will only compress esophageal and cardiac varices (Figure 13.4). The Linton type is easier to use in daily practice. It is effective in bleeding control but rebleeding after balloon deflation is very common. Complications can be serious and include aspiration pneumonia, laceration of the esophagus, malposition in the airway, and can be lethal [8,11,42]. Presence of the balloon for more than 24 hours can cause necrosis of the esophageal or the stomach wall [40]. Figure 13.3 Linton balloon tamponade. A Linton balloon is made of a pear-formed balloon placed over a tube with three lumens. At the proximal side of the tube, three ports are visible: one for inflation of the gastric balloon, one for the esophageal port, and one for the gastric port. Inflate the balloon to check for leaks. Deflate the balloon. Lubricate the tube. Insert the tube, in a ventilated patient, through the nasal (or oral) passage and advance it until it reaches the stomach. Inflate the balloon with 200 mL of air. Withdraw the balloon gently until resistance is felt. This indicates that the gastric balloon is positioned against the cardioesophageal juncture and held by the diaphragm. Now, correct position must be confirmed by a chest X-ray (including the subdiaphragmatic region). If the balloon is in a correct position (under the diaphragm), inflate the Linton balloon to 500 mL. Record the amount of air inflated in the patient’s file. The insufflation port should be clamped to avoid uncontrolled air loss. Finally, attach a rope to the tube and adjust a serum bag of 200 mL on this rope. Place this over a pulley system to ensure traction on the rope and ascertain the correct position of the balloon against the gastroesophageal junction by another chest X-ray. The gastric balloon should not be maintained for more than 24 hours. Figure 13.4 Sengstaken–Blakemore tamponade. A Sengstaken–Blakemore balloon contains two balloons (one esophageal balloon and one gastric balloon) placed over a tube with four lumens: one lumen for every balloon, and two lumens that aspirate the gastric and esophageal contents at the proximal end. Inflate the balloons to check for leaks. Deflate the balloon. Lubricate the tube. Insert the tube in a ventilated patient through the nasal (or oral) passage and advance it until the stomach. Inflate the gastric balloon with 50 mL of air. Withdraw the balloon gently until resistance is felt. This indicates that the gastric balloon is positioned against the cardioesophageal juncture and hold by the diaphragm. The correct position now must be confirmed by a chest X-ray including the subdiaphragmatic region. If the balloon is in the correct position, inflate the esophageal balloon to 150 mL of air maximum and the gastric balloon to 250 mL of air maximum. A manometer can be used to check the air pressure in the esophageal ballon, which should not exceed 40 mmHg [87]. Record the amount of air inflated in the patient’s file. All insufflation ports should be clamped to avoid uncontrolled air loss. Finally, attach a rope to the tube and adjust a serum bag of 200 mL to this rope. Place this over a pulley system to ensure traction on the rope and ascertain the correct position of the balloons in the esophagus and against the gastroesophageal junction by another chest X-ray. The gastric balloon should not be maintained for more than 24 hours. The esophageal balloon may be deflated every 8 hours for 30–60 minutes to prevent esophageal mucosal necrosis. Evidence-based guidelines are lacking. A covered removable esophageal stent has been developed for esophageal varices but has not yet been validated in large trials [43].
Varices, Portal Hypertensive Gastropathy and GAVE
Variceal Bleeding
Diagnosis and Management
Initial evaluation
General Management
Resuscitation
Vasoactive Medication
Antibiotics
Coagulation Disorders
Endoscopy
Esophageal Varices
Gastric Varices
Rescue Treatment in Esophageal and Gastric Variceal Bleeding
Balloon Tamponade
Transjugular Intrahepatic Portosystemic Stent Shunt
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








