Publication
Type of sling
Number of patients
Mean/median follow-up (months)
Definition of success
Success rate (%)
Improved (%)
Explantation rate (%)
Hoda et al.
ATOMS
99
18
0 pads/day and <10 ml in 24 h pad test
63
29
4.0
Seweryn et al.
ATOMS
38
17
0–1 pad/day and <15 ml in 24 h pad test
60.5
23.7
15.8
Bochove-Overgaauw et al.
Argus classic
95
27
Dry or improved
72 (0 pads: 40 %)
18
11
Dalpiaz et al.
Argus classic
29
35
0–1 small safety pad/day
17
Not provided
35
Hübner et al.
Argus classic
101
25
≤1 ml in 20 min pad test
79.2
Not provided
15.8
Romano et al.
Argus classic
48
45
0 pads/day
66
13
10.4
Bauer et al.
ArgusT
42
28.8
0–5 ml in 24 h pad test
61.9
26.2
11.9 % (including failed patients)
Sousa-Escandon et al.
Remeex
51
32
≤1 pad/day
64.7
19.6
5.9
Remeex
The risk of erosion seems to be linked to the degree of compression of the urethra and to infections. De novo urgency seems to be associated with mild postoperative obstruction.
Compared to retrourethral slings postoperative pain is more common after implantation of adjustable sling systems. Early postoperative pain is very common and even persistent pain can occur in up to 5 % of the patients.
Mode of Action
Permanent increase of urethral resistance 10–15 cm H2O to support basis continence.
Argus Classic/ArgusT
The Argus system (Promedon, Argentina) consists of a radiopaque cushioned system with a silicone foam pad for soft compression of the bulbar urethra. Two silicone columns formed by multiple conical elements are attached to the silicone foam and allow system readjustment while two radiopaque silicone washers allow regulation of the desired tension (Fig. 4.1a, b). During implantation measurement of the retrograde leak point pressure (RLPP) is recommended. During intraoperative tensioning the silicone arms are tensioned through the washers until a RLPP of pre-adjustment RLPP + 10 cm H2O is achieved (recommended maximum intraoperative RLPP 40 cm H2O).
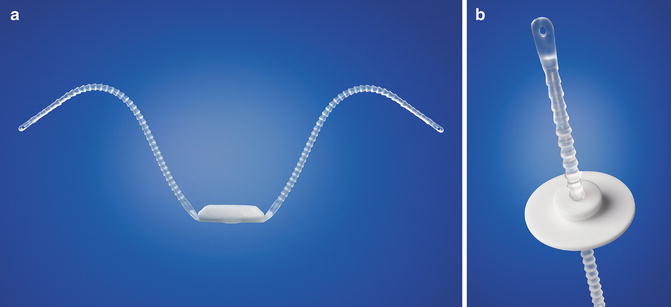

Fig. 4.1
(a) Argus system (with permission of Promedon, Argentina; copyright Promedon). (b) Argus Washer (with permission of Promedon, Argentina; copyright Promedon)
The Argus sling can be implanted via a retropubic (Argus classic—Fig. 4.2) or a transobturatoric (ArgusT—Fig. 4.3) approach.
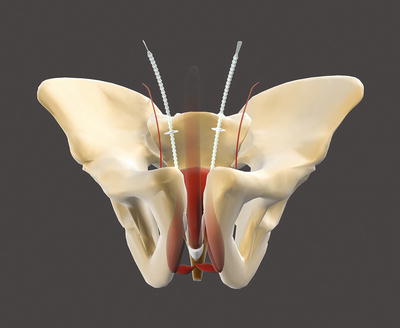
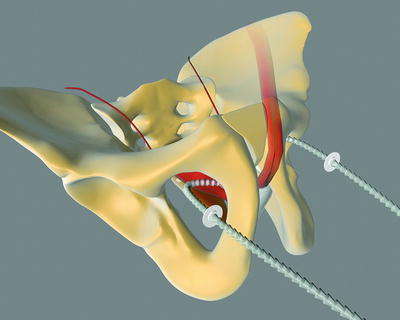

Fig. 4.2
Argus classic (with permission of Promedon, Argentina; copyright Promedon)

Fig. 4.3
Argus T (with permission of Promedon, Argentina; copyright Promedon)
For readjustment the suprapubic incision (Argus classic) respectively the right and left inguinal incisions (ArgusT) have to be re-opened under local or general anesthesia to explore the washers to increase or decrease the tension.
For the Argus classic several published studies with a mean follow-up period of up to 45 months are available. Reported cure rates are 17–79 %.
In 48 patients with mild-to-moderate SUI and a mean follow-up of 45 months (range 36–54 months), a dry rate of 66 % was achieved [7]. Another study evaluated 101 patients with moderate to severe SUI. After a mean follow-up of 2.1 years (range 0.1–4.5 years) a dry rate of 79.2 % (pad test of 0–1 g) was reported [8]. A retrospective study including 95 patients with severe SUI showed success rates (cured + improved) of 72 % and a dry rate of 40 % (0 pads) in a median follow-up of 27 months (range 14–57 months) [9].
Readjustments are required in approximately one third of the patients.
Data concerning irradiated patients are controversial showing equal [8, 10] or lower success rates [9] compared to non-irradiated patients. In addition, one study showed reduced efficacy in patients after treatment for urethral strictures or bladder neck stenosis before sling implantation [9]. Other studies showed no difference or did not evaluate these patients in detail.
Reported intraoperative complications of the Argus classic are bladder injuries (up to 10 %).
Main postoperative complications in a mean follow-up period of up to 45 months are: infections (up to 7 %), significant postoperative perineal pain (up to 27 %), urethral or bladder erosion (up to 13 %), de novo urgency (up to 14 %), retention (up to 35 %), suprapubic extrusion of the columns (up to 7 %), system dislocation (up to 7 %), and explantation of the system (up to 35 %) due to complications (e.g., infection, erosion, pain) [7–10]. In the early postoperative period urinary retention can occur (up to 35 %). Moderate adjustment of the sling during implantation (recommended maximum intraoperative retrograde leak point pressure <40 cm H2O) seems to reduce the complication rate especially of postoperative retention and sling erosion [8].
Detailed information concerning late postoperative complications is rare. In up to 3 % persistent pain occurs and there is one reported case of explantation after several months due to persistent pain [10]. In general, for the first generation of the Argus classic more complications are reported [10].
The implantation technique of the ArgusT was first published in 2004 including one case report [11]. Until now, only one study with a mean follow-up of 28.8 months including 42 patients is published [3]. Dry rate with a pad test of 0–5 g/24 h was 61.9 %. 26.2 % of the patients had a reduction of incontinence of at least 50 % and five patients failed. In all patients the ArgusT was explanted and an artificial sphincter (AMS 800, American Medical Systems, USA) was successfully implanted. Subgroup analysis showed no difference in outcome of patients with additional radiotherapy, on patients with preoperative urine loss <500 g/24 h and >500 g/24 h and in patients with and without anti-incontinence pretreatment. In addition, type of prostate surgery, age, and BMI had also no impact on outcome.
Median adjustment rate was 1.7.
There were no intraoperative complications or postoperative urinary retention reported.
The following postoperative complications occurred: urinary tract infection (2.4 %), perineal wound infection (2.4 %), suprapubic wound infection (4.8 %), postoperative analgesics for >3 months (16.7 %), postoperative urgency (7.1 %), explantation due to persistent pain (4.8 %).
In general, the results of the Argus T seem to be comparable with the Argus classic. However, in contrast to the Argus classic, in the Argus T postoperative pain and persistent pain seems to be higher.
ATOMS
The ATOMS system (A.M.I., Austria) consists of an adjustable cushion fixed with monofilament polypropylene mesh arms around the Ramus inferior of the Os pubis (Fig. 4.4). The mesh arms establish a 4-point fixation. A titanium port for adjustment of the cushion volume is integrated and placed in the left symphysis (inguinal port—Fig. 4.5) or scrotal region (scrotal port—Fig. 4.6). The sling is implanted via a transobturator approach using a perineal single-incision. Compression of the bulbar urethra is achieved via the adjustable cushion.
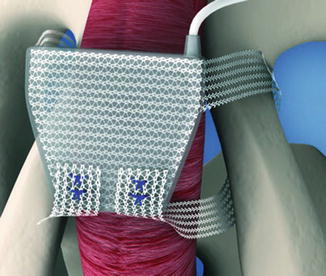
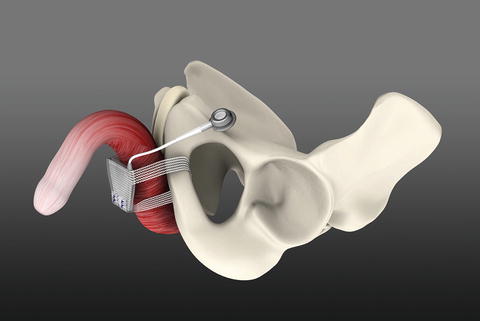
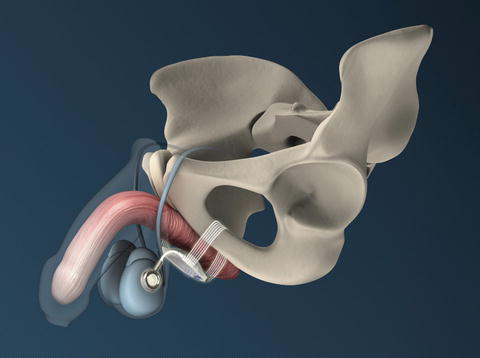

Fig. 4.4
Position of ATOMS (with permission of AMI, Austria; copyright AMI)

Fig. 4.5
ATOMS with inguinal port (with permission of AMI, Austria; copyright AMI)

Fig. 4.6
ATOMS with scrotal port (with permission of AMI, Austria; copyright AMI)
For the ATOMS system, two studies are published. In both studies the ATOMS with perineal port was evaluated.
The first study evaluated 38 patients with a mean urine loss of 747 ml in the 24-h pad test (230–1600 ml) and a mean follow-up of 16.9 months (range 13–21 months) [12]. 60.5 % of the patients were considered dry (0–1 pads and less than 15 ml in the 24-h pad test) and 23.7 % improved (more than one pad and 16 to 100 ml in the 24-h pad test). In 15.8 % of the patients implantation failed (more than two pads daily or more than 100 ml in the 24-h pad test). Mean number of adjustments was 3.97 (range 0–9).
The second study evaluated 99 patients with a mean urine loss of 681 ml in the 24-h pad test [13]. After a mean follow-up of 17.8 months (range 12–33 months) 63 % of the patients were considered dry (0 pads and <10 ml in 24-h pad test) and 29 % were improved (daily pad use reduced by >50 % or patients needed 1–2 pads/24 h and 10–40 ml in 24-h pad test). 8 % of the patients failed. Mean number of adjustments was 3.8 (range 1–6). For every adjustment, 2 ml saline solution was added to the cushion via the port.
No intraoperative complications are published.
Reported postoperative complications are the following: transient perineal or scrotal pain/dysesthesia (up to 68.7 %), early port infections leading to explantation (up to 10.5 %), retention (up to 3 %), explantation due to persistent pain (up to 3 %) and urethral erosion (up to 3 %).
Patients with previous radiotherapy or previous surgery for incontinence show comparable results to non-irradiated/non-pretreated patients without a higher incidence of complications.
The impact of age, comorbidities on outcome or complications is not evaluated.
Remeex
The Remeex system (Neomedic, Spain) consists of a mesh connected via two monofilament traction threads to a suprapubic mechanical regulator and is positioned under the bulbar urethra (Fig. 4.7). The system is implanted via a retropubic approach. The regulation part—the so-called “varitensor”—is a permanent implant (1 × 1 × 2.5 cm cubic device with internal never-ending axis to wind the traction threads) subcutaneously over the abdominal rectum fascia above the pubis. Adjustment is conducted via an external manipulator starting on day 1 after implantation which stays in place after implantation. The external manipulator is removed after first adjustment.
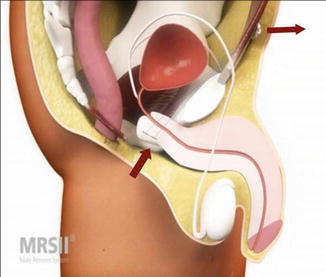

Fig. 4.7
Remeex (with permission of Neomedic, Spain, copyright Neomedic)
Subsequent adjustments are performed under local anesthesia via the rejoined external manipulator to the varitensor.
There are two published studies; however, one of them is only reporting preliminary results of six patients [14]. In a multicentric European Study 51 men with mild-to-severe SUI were treated with the Remeex [15]. The average follow-up was 32 months (range 16–50 months). 64.7 % of the patients were considered cured (no pads: 75.8 %; small pads or sanitary napkins for security reasons but normally dry: 24.2 %) and 19.6 % were improved. In 15.7 % of the patients the implantation failed.
90 % of the patients were adjusted during the early postoperative period; 86 % required a second adjustment between 1 to 4 months after implantation, and 33 % required more than two adjustments.
The only reported intraoperative complication is bladder perforation (up to 10 %).
Reported postoperative complications are transient perineal discomfort or pain in the majority of the patients. In addition, removal of the device (6 %) due to infections of the varitensor or due to urethral erosion is reported. During adjustment a rupture of the monofilament traction threads can occur. In addition, depending on degree of compression residual urine can occur.
The impact of age, comorbidities, or previous radiotherapy on outcome or complications is not evaluated.
Advantages and Disadvantages of Adjustable Sling Systems
Postoperative adjustment is possible—even after years. Tension can be adjusted to patients’ increased postoperative physical activity. Good results are also in severe PPI and irradiated patients possible.
Adjustable sling systems are associated with overall higher complication and explantation rates in comparison to fixed male slings.
Outcome in patients after treatment for urethral strictures or bladder neck stenosis seems to be decreased.
Treatment after Failed Adjustable Sling System
If the continence status is not improved by adjustment salvage treatment should be considered if the patients ask for further improvement.
In general, AUS implantation is the salvage treatment of choice. Several studies showed AUS implantation after failed Argus sling and ATOMS is still possible without specific problems and shows good results without increased complication rate [8, 13, 16]. No data exist concerning salvage treatment after failed Remeex sling implantation. However, data should be comparable to Argus and ATOMS. The adjustable sling systems (especially the non-mesh parts—explantation of the mesh parts can result in damage of the collateral tissue especially the urethra) should be explantated before AUS explantation.
If there is a damage or slippage of the sling system exchange of the system can be considered. In addition, there is one study reporting the implantation of an AdVance sling after failed adjustable sling systems [17]. However, no detailed analysis of this subgroup is available. In general, before considering the implantation of a retrourethral sling after failed adjustable sling system a proper evaluation of the residual sphincter function and mobility of the posterior urethra is necessary. However, implantation of an AdVance sling as salvage treatment salvage treatment cannot be widely recommended and should only be performed in highly selected patients and by an extremely experienced surgeon.
Fixed Male Slings
Fixed male slings can be divided into two types:
Retrourethral slings.
Fixed compressive slings.
Mode of Action
The hypothesized mechanism is not thoroughly understood.
The mode of action of the retrourethral slings seems to be multifactorial:
Correction of postoperative hypermobility of the posterior urethra.
Increased length of functional urethra.
Venous sealing effect.
The mode of action of fixed compressive slings may result from a combination of urethral compression with subsequent permanent increase of urethral resistance and angulation.
Retrourethral Slings
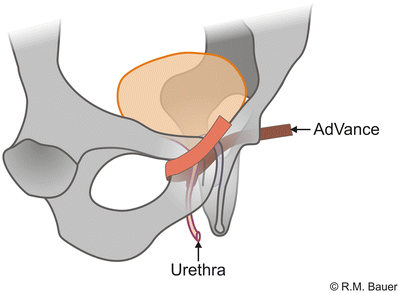
Worldwide, the AdVance sling (American Medical Systems, USA) is the most commonly used sling and the most evaluated sling with a couple of published studies—mainly single-center studies.
The AdVance sling is a retrourethral sling (Fig. 4.8). It consists of a polypropylene mesh and is positioned under the membranous urethra via a transobturatoric approach (Fig. 4.9).
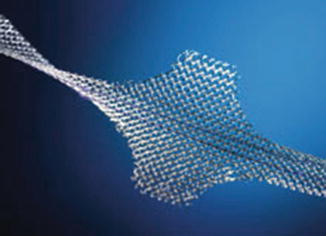

Fig. 4.8
AdVance sling (with permission of American Medical Systems, USA; copyright AMS)
For the correct placement of the sling dissection of the centrum tendineum is necessary. Here the sling is fixed on the bulb and thereafter adjusted. It is postulated that due to intraoperative adjustment the lax and descended supporting structures of the posterior urethra and sphincter region after prostate surgery are relocated into the former pre-prostatectomy position (Figs. 4.10a, b) [19, 20]. Several urodynamic studies suggest that the AdVance sling does not cause any obstruction to the urethra [20–22].
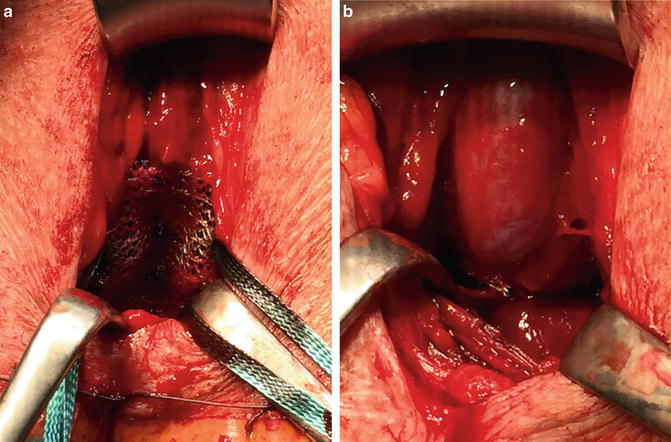

Fig. 4.10
(a) AdVance sling before adjustment. (b) AdVance sling after adjustment
For the AdVance sling cure rates of 9–63 % with a follow-up of up to 40 months are published [17, 23–30]. However, efficacy rates are difficult to compare due to heterogeneous definition for cure varying from 0 to 1 pad/day or different amounts of daily urine loss in pad tests (1-h pad test or 24-h pad test).
Two prospective studies with a 3 year follow-up including one multicentre study are available. The multicentre study included 156 patients and reports a cure rate of 53 % (no pad or one security pad) and a success rate of 77 % (reduction of daily pad usage ≥50 %) [29]. No worsening over time occurred.
The second study included 30 patients and reported a cure rate of 60 % (no pad usage or one security pad) and a success rate of 73 % (reduction of daily pad usage ≥50 %) [28].
There are several risk factors for failure under discussion. Incontinence severity and residual sphincter function including ability to close the sphincter completely and functional sphincter lengths (≥1 cm) seem to have a positive effect on efficacy [19, 27, 29, 31, 32]. A Valsalva leak-point pressure of >100 cm H2O, representing a good residual sphincter function and a lower degree of incontinence, is associated with better efficacy [33].
Surgery for urethral stenosis or anastomotic stricture between prostatectomy and sling implantation is significantly associated with lower dry rates [27].
In irradiated patients, the AdVance sling shows reduced treatment success with dry rates between 18 and 53 % [29, 34–36].
Previous surgical treatment may affect negatively the efficacy, especially if scarring of the surrounding tissue of the bulb occurred [28, 29]. However, there is one study reporting the outcome of AdVance sling implantation in 19 patients with recurrent stress incontinence after implantation of an artificial sphincter [37]. In all patients etiology of the recurrent stress incontinence was urethral atrophy. In all patients the AdVance sling was implanted in addition to the artificial sphincter. After a mean follow-up of 13 months 79 % were dry using no pads and 21 % were improved using one pad per day. 53 % of the dry patients were dry without reactivation of the artificial sphincter and 47 % maintained dry with a combination of the AdVance sling and the activated artificial sphincter. In these patients no intraoperative or postoperative complications (e.g., infection, retention, de novo urgency) occurred.
In contrast, surgeon’s experience (>25 AdVance sling implantation) may have a positive effect on outcome [32].
Severe complications including explantation or infection of the AdVance are rare.
The main postoperative complication is transient acute postoperative urinary retention (up to 21 %) requiring temporary recatheterization and mild postoperative perineal pain [17, 23–30, 38].
Two intraoperative injuries of the urethra are reported [38–40]. No further intraoperative complications are published.
Reported main early postoperative complications are transient acute postoperative urinary retention (up to 21 %) requiring temporary recatheterization, mild dysuria in 14 %, mild transient perineal or scrotal pain in 0–20 %, local wound infection 0.4 %, severe wound infection leading to sling explantation in 2.8 % (n = 1), urinary infection with fever 0.4 % and perineal-adductor compartment haematoma under anticoagulation (n = 1) [24, 27–29, 38, 41]. In addition, there is a report about one sling infection in an irradiated patient 5 months after second AdVance sling implantation resulting in explantation of both slings [42]. In general, explantation rate is very low with a very low number of reported cases in total (<10).
There are several studies dealing with late postoperative complications and were shown to be uncommon. However, the following late complications are reported: urinary retention for up to 5 months (n = 2; 5.7 %), persistent retention due to sling slippage leading to sling incision (n = 1; 0.4 %), persistent perineal pain (n = 1; 0.4 %), significant adductor muscle pain (n = 2; 1.7 %) and symphysitis (n = 1; 0.4 %) leading to sling explantation—probably symphysitis was not induced by the sling [27–29, 38, 43].
The impact of age, comorbidities or previous radiotherapy on intraoperative or postoperative complications is not evaluated.
In end of 2010, the second generation—AdVanceXP– was introduced (Fig. 4.11). The AdVanceXP is not available in the USA (no FDA approval).
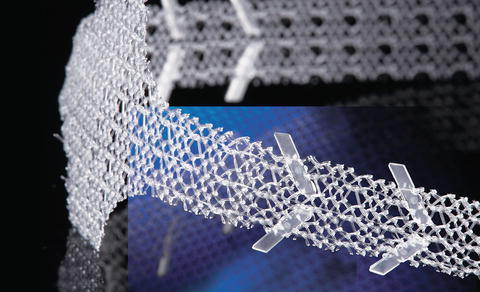

Fig. 4.11
AdVance XP sling (with permission of American Medical Systems, USA; copyright AMS)
The AdVanceXP addresses several important issues:
Anchors along the sling arms to reduce early failure due to sling loosening or slippage.
A new needle shape for easier tunneling especially in larger or more obese patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree