14
Treatment of the complications of gastro-oesophageal reflux disease and failed gastro-oesophageal surgery
Introduction
Gastro-oesophageal reflux affects up to a quarter of the UK population on a regular basis. Most will not seek medical help and self-medicate. Of those who do see a doctor the vast majority will be well controlled by medical therapy – invariably proton-pump inhibitors. It has been calculated that only 1% of patients with gastro-oesophageal reflux disease (GORD) will develop complications of the reflux. These complications are consequent on damage to the mucosa resulting in erosive oesophagitis with, rarely, a peptic oesophageal ulcer, a peptic stricture or Barrett’s oesophagus (see Chapter 15). The vast majority of sufferers of GORD have non-erosive reflux disease (NERD) and never develop either erosive oesophagitis or Barrett’s oesophagus.1
There are two main treatments for GORD. These are medical therapy with proton-pump inhibitors or surgical therapy based on either a total or partial fundoplication. Comparative studies suggest there is little to choose between the two therapeutic arms in either the control of symptoms or prevention of complications. This is well documented in the results of the multicentre LOTUS trial, comparing esomeprazole and laparoscopic antireflux surgery, with good control of symptoms and microscopic oesophagitis in over 85% of cases in both arms out to 5 years.2,3
In recent years there has been a significant increase in the use of fundoplication based on the laparoscopic approach. This is claimed to be a one-off long-term treatment that removes the need for expensive long-term ingestion of proton-pump inhibitors. However, it is recognised that with time the use of these drugs does increase in patients who have had a successful initial surgical outcome, reaching 25% at 10 years.4,5
In the USA, the number of antireflux operations increased by 260% between 1993 and 2000 but decreased by 40% between 2000 and 2006.6 Similar trends have been seen in Europe, particularly in Sweden. The reasons are unclear but may reflect disappointing long-term results for surgery, with a significant proportion of patients back on medical therapy as described above and recognition of the complications, which although rare can have a serious impact on a young, previously fit patient.
Gastro-oesophageal reflux results from failure of the lower oesophageal sphincter to either provide a mechanical barrier (volume/supine refluxers) or which relaxes inappropriately, usually secondary to gaseous distension of the gastric fundus (upright refluxers). These differences can have an effect on the outcome of antireflux surgery whereas defective oesophageal motility, other than undiagnosed achalasia, does not.7,8
Complications of GORD
Complications arise from damage caused by the refluxate to the squamous mucosa of the oesophagus. The contents of the refluxate may include any combination of acid and/or ‘bile’ (duodenogastric contents). Chronic exposure of the oesophageal mucosa to this fluid in patients with an intact stomach causes inflammation, can result in erosive oesophagitis with ulceration and subsequent peptic stricture, and in some results in metaplastic change and the development of Barrett’s oesophagus. It appears that acid alone is the principal factor in determining the severity of oesophagitis, whereas in patients experiencing symptoms who are on long-term proton-pump inhibitors weak acid or bile is implicated.9,10
Short oesophagus
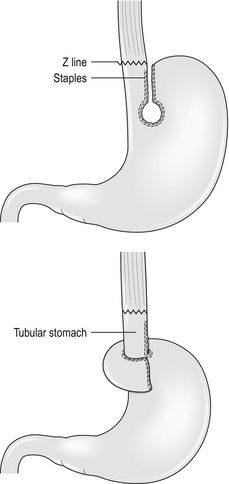
Those who recognise the complication advocate a Collis gastroplasty to lengthen the oesophagus through either an open abdominal or laparoscopic approach. It may provide a better outcome in such patients when compared with fundoplication alone.11 In this procedure a 40 F bougie is passed into the stomach and kept on the lesser curve. A neo-oesophagus is created using a circular and linear cutting stapler and a loose wrap performed using the mobilised stomach (Fig. 14.1). This has the potential to leave a tube of acid-secreting stomach above the wrap, with consequent complications of bleeding, ulceration and stenosis. It has been used in revisional surgery, where mobilisation of the gastro-oesophageal junction below the diaphragm has proven difficult. In experienced hands the results are reported to be good.12
Gastrointestinal haemorrhage
The treatment of erosive oesophagitis is medical with proton-pump inhibitors in the first instance, with excellent healing rates, although double-dose treatment may be required with maintenance therapy after healing has been achieved.13 To attribute gastrointestinal haemorrhage to erosive oesophagitis is a matter of exclusion after full endoscopic examination as it is not a common cause of significant bleeding except in those with a tendency to bleeding, usually as a result of other medication they are taking.
Peptic oesophageal stricture
The incidence of symptomatic stricture is extremely low in the pantheon of reflux disease. The management involves endoscopy and biopsy to exclude serious pathology followed by optimum acid inhibition with proton-pump inhibitors and H2-receptor antagonists and gentle dilatation with an endoscopic balloon (Fig. 14.2). The diameter is dependent on the size of the stricture but it is better to undertake sequential dilatation starting with a 10-mm balloon rather than use too big a balloon and risk splitting the oesophagus. The risk of perforation is of the order of 2–3%. This should be recognised at the time and treated conservatively with nil by mouth, intravenous proton-pump inhibitors and antibiotics plus placement of a nasojejunal tube under screening for feeding. Success can be achieved in over 90% of cases with conservative management. If the perforation is not recognised and the patient develops sepsis then a more aggressive approach is required, including drainage and surgical repair. Such patients are best referred to specialist centres with full intensive care and interventional radiological support.14,15 The use of self-expanding plastic stents in such perforations shows promise but can cause problems and is best avoided. Any stent used in this context must be removable.
Patients with symptomatic peptic strictures will often require repeat dilatation to maintain swallowing. In such cases injection of steroids can be beneficial and division of a tight band-type stricture with a laser can reduce the frequency of dilatation.16 The use of self-expanding plastic stents is controversial and in resistant strictures only removable stents (plastic) or the new biodegradable stents should be used.17 The patient with a stricture resistant to these treatments may require surgical resection. Such cases may be technically difficult as there has been transmural inflammation or perforation and an open approach is recommended. An algorithm is shown in Box 14.1.
Failed antireflux surgery
Failure may be due to persistence or recurrence of reflux symptoms, development of new symptoms or complications of the surgical procedure. The incidence of complications17–19 is listed in Table 14.1. Complications and failures tend to occur soon after laparoscopic surgery and later after open surgery.
Table 14.1
Complications of antireflux surgery
| Pneumothorax | 2% | |
| Paraoesophageal hernia | 7% | |
| Dysphagia – Slipped wrap, tight wrap, tight hiatus | early late | 34% 6% |
| Perforated oesophagus | 1% | |
| Bloating/diarrhoea | 30% |
As described above, the success rate for fundoplication in controlling symptoms is in the region of 85% using accepted criteria for surgical success – volume reflux, failed medical treatment, etc.2–5 This implies that 15% fail and with time even successful initial surgery results in 25% of patients subsequently requiring regular medication.
It is now recognised that the type of wrap (partial or total) does not have a long-term effect on dysphagia, although total fundoplication may give better control of reflux symptoms whereas partial fundoplication has less dysphagia in the short term, although this difference disappears with time.20,21
Investigation of the failed antireflux operation
Barium studies
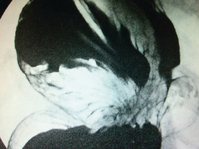
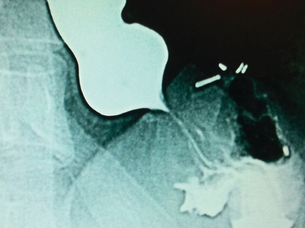
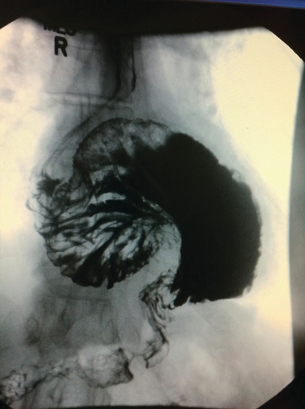
Barium studies provide important information about both anatomy and function. A swallow can demonstrate the pattern of obstruction and its relation to the diaphragm. It can also demonstrate the integrity of a wrap and slippage into the chest (Figs 14.3 and 14.4), which is not infrequently associated with volvulus (Fig. 14.5). It is also important to look at the gastric component of the study to visualise gastric emptying and pyloric function.
Computerised tomography
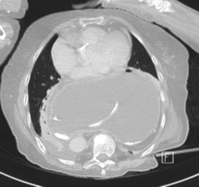
CT scanning with contrast should be undertaken in all cases where malignancy is suspected with endoluminal ultrasound scan if there is doubt. It is also invaluable where there is a large intrathoracic stomach to display the anatomy, especially if the repair is to be undertaken using the transabdominal approach (Fig. 14.6). CT is almost invariably contributory in difficult revisional cases.
Oesophageal physiology tests
Repeat oesophageal physiology and in particular oesophageal impedance can be very revealing in failed surgery, especially if it was not undertaken prior to original surgery. The missed diagnosis of achalasia will invariably result in dysphagia, as will scleroderma where the oesophagus is amotile. There is no evidence, however, that normal but low-amplitude motility affects outcome, and high-amplitude waves may be associated with a tight wrap.8
The results of 24-hour pH monitoring post-fundoplication are very interesting.22 The usual pattern is that there is no measurable reflux in spite of symptoms that seem classical of reflux. This relates to the observation that many patients undergoing revisional surgery for recurrent symptoms have a wrap that is in good position and is intact. The cause of the symptoms is therefore unclear and caution must be expressed on the successful outcome of re-operation. When the pH studies are positive and there is good symptom correlation and positive DeMeester score, this will usually be associated with wrap disruption and a better outcome can be expected with revision.
Management of failure after antireflux surgery
Recurrence of reflux symptoms
Recurrence of symptoms as described earlier is more common than generally recognised and a significant proportion of patients (25%) are back on medication post-surgery, although their symptoms are better controlled. It is wise to repeat the physiology studies in such patients to identify those who have true reflux, especially if considering revisional surgery.23
The reasons for this recurrence of symptoms include wrap disruption, wrap slippage and migration into the chest. The latter is more frequent when at the first operation there was a large hiatal defect, which was not closed properly with sutures, and if the sac was not excised from the chest or the repair was associated with tension (Fig. 14.7). Closure of a large hiatus with removal of the sac can be difficult, especially when undertaken laparoscopically, and if problems arise this should lead to conversion to open surgery. An alternative is to close the hiatus with a mesh, preferably from behind the oesophagus. Good results have been reported using this technique but caution is needed as the mesh can erode the oesophagus and cause dense strictures at the hiatus.23,24 Removal will invariably require open surgery, usually via a thoracoabdominal incision with an oesophagogastrectomy. The use of man-made products to cover the hiatus adjacent to the oesophagus should be avoided if at all possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree