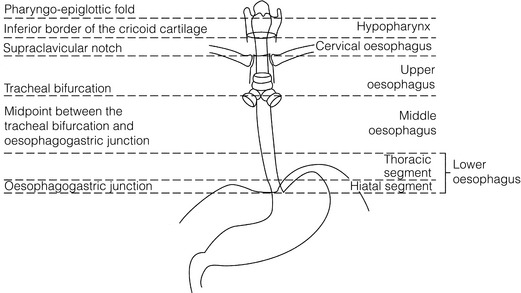
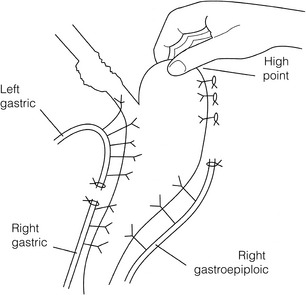
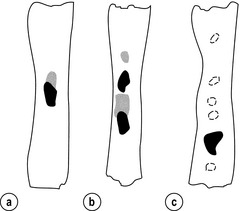
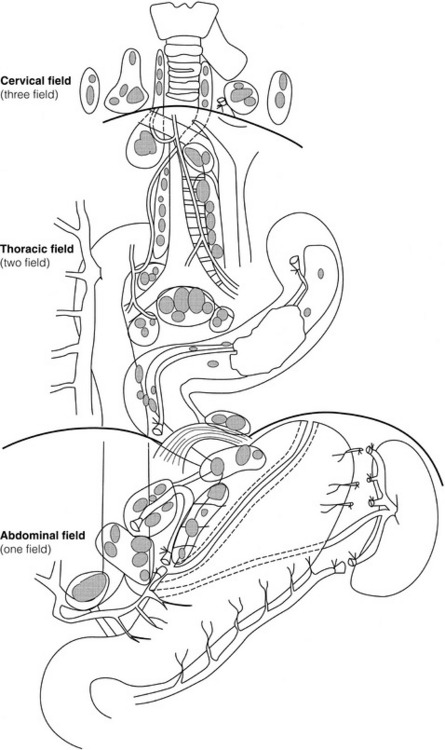
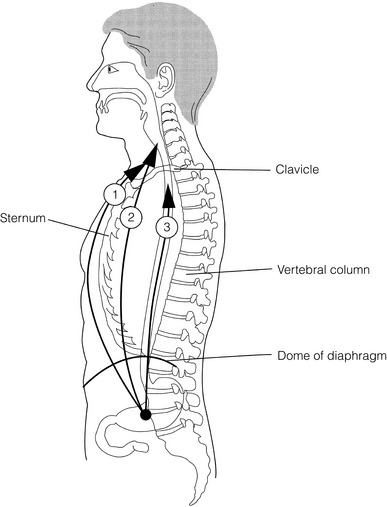
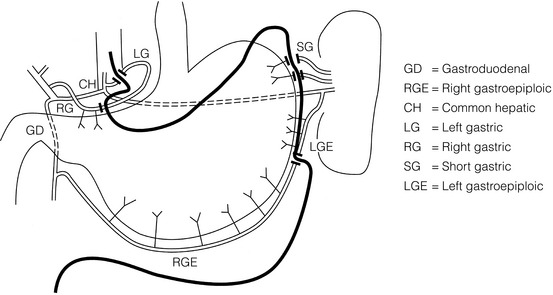
5 While treatment for cancer of the oesophagus is multidisciplinary, surgery is still the primary mode of therapy. In the UK, 70% of patients now present with adenocarcinoma of the lower oesophagus or gastro-oesophageal junction, which represent a different disease from the previously more common squamous carcinoma. This chapter discusses the surgical management of adenocarcinoma of the lower oesophagus and the cardia (Siewert types 1 and 2), which are frequently staged and treated as oesophageal cancers, but not subcardial tumours (Siewert type 3), which are described elsewhere (Chapter 7). Unfortunately, the disease often presents late when increasing dysphagia has developed over several months. As a result of poor fitness or unresectable disease only 30–40% of patients are suitable for radical, potentially curative treatment, whilst the majority receive non-surgical therapies with the aim of palliation. Outcome is strongly stage dependent; whilst early tumours have excellent results with surgery alone, the majority with transmural or node-positive tumours benefit from multimodality therapy, combining surgery with neoadjuvant chemotherapy or chemoradiotherapy (Chapter 9). The multidisciplinary team must exercise judgment in the choice of the appropriate combination of therapies for each individual patient. This will depend on patient age, fitness, symptoms, prognosis and evidence base, as well as the overall stage and histopathology. Squamous cell carcinoma arising in the cervical and thoracic oesophagus and adenocarcinoma arising in the thoracic oesophagus and cardia differ in their mode of spread and response to therapeutic modalities. It is essential that the anatomical divisions of the oesophagus are described such that the different therapeutic surgical procedures adopted for tumours at each site can be understood (Fig. 5.1). The TNM classification (Version 7, released in 2009)1 combines the salient features of the staging process. This classification has divided the oesophagus into discrete anatomical regions (Fig. 5.1) and is described in Chapter 2. The lymphatics of the oesophagus are distributed predominantly in the form of a submucosal plexus and a paraoesophageal plexus. Both plexuses receive lymph from all parts of the respective layers of the oesophageal wall. The plexuses communicate through penetrating vessels that traverse the longitudinal and circular muscle walls. The paraoesophageal plexus drains into the paraoesophageal lymph nodes, which are situated on the surface of the oesophagus, and also into perioesophageal lymph nodes, situated in close proximity to the oesophagus. Lymphatics also drain from the perioesophageal nodes to the lateral oesophageal nodes or directly from the paraoesophageal to the lateral oesophageal nodes, skipping the perioesophageal group2 (Box 5.1). Meticulous preoperative evaluation to accurately stage the tumour and estimate surgical risk is a crucial prerequisite to successful surgical outcome in this disease (see also Chapters 3 and 4). Malnutrition is associated with loss of tissue function, leading to many potential complications during the postoperative period, such as wound breakdown, respiratory failure secondary to poor respiratory muscle function, deep vein thrombosis and infective complications.3 Perioperative enteral nutrition with the addition of nutrients that can modulate the immune system, termed ‘immunonutrition’, has been proposed to further reduce postoperative complications and improve outcome.5 Placement of a feeding jejunostomy at the time of surgery is routine in many units. Although mortality related specifically to feeding jejunostomy is less than 1%,6 it is not without morbidity, both in-hospital and longer term (from adhesions). Nevertheless, establishing enteral nutrition in patients with complications following oesophagectomy, when they are most catabolic septic and ill, is a major therapeutic problem and avoiding re-operation has significant advantages. Routine preoperative and postoperative feeding by jejunostomy in every patient has yet to be proven efficacious on current evidence.7 Furthermore, a recent randomised controlled study comparing standard perioperative nutrition with immunonutrition failed to demonstrate any clinical advantage.7 A variety of other routes of nutrition have been assessed, with a recent systematic review showing no strong direct evidence to support one particular route.8 Nasojejunal and nasoduodenal tubes are associated with a significant rate of dislodgement.8 Optimisation of respiratory function is vital in preventing serious pulmonary complications associated with prolonged surgery and thoracotomy (see Chapter 4). Smoking must be stopped as early as possible, ideally 6 weeks prior to surgery, with the aid of nicotine replacement. Preoperative physiotherapy with coughing exercises and effective use of the diaphragm by restoration of muscle strength through ambulation is encouraged. High-risk patients should also be provided with vigorous physiotherapy with or without bronchodilators prior to surgery. Orodental hygiene should be undertaken, removing any source of chronic sepsis that could disseminate infection to the tracheobronchial tree during intubation. Many surgeons advocate routine use of an incentive spirometer; they are inexpensive, and used properly they help to set goals for patients that can be measured, albeit indirectly. Survival is related to the stage of disease. Patients with stage I disease can expect a 5-year survival of greater than 80%,9 emphasising the importance of early detection. Resection alone, therefore, must be the chosen method of therapy in fit patients with T1 tumours of the middle and lower thirds of the oesophagus. In stage III disease, surgery alone produces poor results with prolonged survival for only 10–20% of cases.10 It appears that both neoadjuvant chemotherapy and radiotherapy provide a benefit for these patients.11 Further randomised trials must be completed in order to outline the optimal therapeutic strategy. Survival following surgical resection for all stages of tumour has improved over the past 20 years, with morbidity and mortality falling. The reasons for this are listed in Box 5.2 and were well described in the COG Guidance Report on Upper GI Cancers.12 Many studies have confirmed that results parallel experience in managing this condition,13 and poor results occur when experience is limited.14,15 There is now overwhelming evidence to confirm the influence of surgeon case volume on the outcome of site-specific cancer surgery.14–16 Centralisation of oesophagogastric resection in specialist units in the UK has provided sufficient caseload to support strong multidisciplinary teams. Other reasons for improved outcome include better patient selection, earlier diagnosis by open-access endoscopy, surveillance of Barrett’s oesophagus, and improved preoperative, operative and postoperative management. The majority of authors favour a subtotal oesophagectomy to optimise longitudinal margins and take into account submucosal spread of both squamous and adenocarcinomas. There has been much debate around what length of macroscopically normal oesophagus to allow for complete resection margins. In squamous carcinoma this pertains especially to the proximal margin, whereas in adenocarcinoma the distal margin (gastric) is usually the greater concern. Skinner17 advocated a minimum resection margin of 10 cm from the palpable edge of the tumour. However, this figure does not take into account the nature, location and pattern of occurrence of the primary cancer. Neither does it discriminate between in vivo margins and margins measured by the histopathologist after shrinkage has occurred during fixation.18 Primary tumours with multicentric lesions require more extensive longitudinal margins. In squamous cancers, three representative patterns of presentation are encountered (Fig. 5.2).19 Failure to take these into account may explain high R1 rates (40%) when the oesophageal resection margin is limited to only 4 cm; compared to this, R1 was 17% when the margin was 10 cm. A 10-cm resection margin is a goal to attain in both directions if this is possible. In practice, this rule can rarely be achieved. A 10-cm margin either side of a 6-cm tumour would require an overall length of specimen exceeding that of the normal human oesophagus. It is the authors’ opinion that when only a short resection margin can be obtained through the thoracic exposure, a cervical phase with near total oesophagectomy is advisable. Figure 5.2 (a) A single cancer. (b) Multifocal cancer. (c) Intramural lymphovascular spread. There is a high risk of positive resection margins in (b) and (c). Shaded areas represent submucosal spread. Adenocarcinoma of the lower oesophagus commonly infiltrates the gastric cardia, fundus and lesser curve. Extensive sleeve resection of the lesser curve and fundus with the formation of a tubular conduit is necessary to minimise positive distal resection margins. Other studies have demonstrated that patients with microscopically positive margins undergoing palliative resection died of other manifestations before clinical evidence of locoregional recurrence.20,21 A tumour-free surgical margin is therefore not the only important factor to be considered in radical surgery. Nevertheless, it should remain the main goal of every operation. A clear radial resection margin is equally important and is accepted as an independent prognostic factor for oesophageal cancer, with a definition of R1 being less than 1 mm clear margin.22,23 The potential benefits of extended lymphadenectomy, discussed later, only pertain if the primary tumour has been completely excised (R0). Radical en-bloc resection techniques, outlined below in operative description, aim to produce a clear radial resection margin. Roder et al.24 showed a statistically significant difference between R0 and R1 (microscopic residual disease) or R2 (macroscopic residual disease) resections for squamous cell carcinoma in a series of 204 resections with 5-year survival rates of 35% and < 10%, respectively. Lerut et al.25 demonstrated a 20% 5-year survival for R0 vs. zero 5-year survival for R1 and R2 resections in advanced stage III and stage IV adenocarcinomas and squamous cell carcinomas. Lymph node involvement is another independent variable for prognosis, in terms of both locoregional recurrence and survival. Patients with higher nodal burdens have worse outcomes after resection. The Worldwide Esophageal Cancer Collaboration has used pooled data from centres that have undertaken radical lymphadenectomy to report in detail the relationship between nodal involvement and survival.26 Lymph node tiers draining oesophageal cancer have been described according to the anatomy of the lymphatic drainage of the oesophagus.2,27,28 The extent of lymphadenectomy associated with resection of these tiers is demonstrated in Box 5.1 and Fig. 5.3. Figure 5.3 Extent of resection and fields of lymph node dissection routinely carried out for cancer of the oesophagus. The fields of nodal dissection should not be confused with the histopathological staging of nodal involvement (see Chapter 3, Table 3.3). In the past some authors felt that lymph node metastases were simply markers of systemic disease and their removal conferred no benefit.23 Others contended that cure could be obtained in some patients with positive nodes by a radical lymphadenectomy with clear resection margins.29 There has been increasing support for radical lymphadenectomy over the last 5 years.30 Optimal staging: Radical lymphadenectomy allows more accurate pathological staging.25,31–32 TNM7 relies not only on the finding of positive lymph nodes, but on how many are found (N1, 1–2; N2, 3–6; N3, ≥ 7). Without an adequate lymphadenectomy, the patient is deprived of the most accurate prognosis available, and the patient’s unit is deprived of a quality benchmark. Quality control is not possible as the baseline information, accurate pathological stage, is missing. If an inadequate lymphadenectomy is undertaken, survival will be worse than predicted for stage (the phenomenon of stage migration). Locoregional tumour control: Locoregional tumour control is an important goal in treating oesophageal carcinoma; recurrent locoregional mediastinal disease can be very difficult to palliate. As Lerut et al. emphasise, long-term palliation is a significant benefit for those patients who are not cured by surgery.25 It is impossible to separate potential benefits of radical lymphadenectomy from radical en-bloc resection, as already mentioned. Radical en-bloc resection with lymphadenectomy has been associated with prolonged tumour-free survival; this is partly a result of consistently clear resection margins and partly a result of complete removal of involved nodes. Nodal and local tumour recurrence is less common after radical en-bloc resection in a number of retrospective series.25,31,33–35 Single-institution comparative studies have also shown better survival.36,37 Altorki et al. described a local recurrence rate of only 9.7% and a 33% 5-year survival for node-positive oesophageal cancer patients using a three-field lymphadenectomy and en-bloc resection.38 Locoregional recurrence may be further reduced by induction therapy, whether chemotherapy alone or chemoradiotherapy, with fewer positive nodes as well as an increase in R0 resection, consistent with improved disease-free and overall survival in some randomised trials.39 Improved cure rate: It is extremely difficult to demonstrate that radical lymphadenectomy improves cure rate in a conventional randomised controlled trial. Patients allocated to less than radical lymphadenectomy would be understaged, so no baseline would exist and the groups would not be comparable. Furthermore, most patients now have induction therapy that changes their nodal status during treatment. There are therefore very few randomised trials in the literature.40–42 Despite the limitations, there are indications from the Dutch trial,40,43 comparing radical transthoracic and transhiatal resection, that patients with a limited number of positive nodes (1–8) had a significantly better survival following radical transthoracic oesophagectomy compared with less radical transhiatal resection. This group of patients would be expected to benefit from extended lymphadenectomy if it conferred survival advantage. Node-negative patients did well and those with a higher nodal burden did poorly, irrespective of the radicality of surgery (see Fig. 5.4). Another smaller, retrospective comparison showed improved survival with radical en-bloc resection compared with transhiatal resection in patients with T3N1 disease with fewer than eight nodes involved.44 Figure 5.4 Three routes of oesophageal reconstruction: (1) presternal route; (2) retrosternal route; (3) posterior mediastinal route. The role of radical lymphadenectomy in early-stage disease remains in question, and depends on the precise depth of invasion of the primary tumour.40 There is some evidence that even patients with early-stage carcinoma, in whom a significant proportion can have nodal involvement, could benefit from extensive resection with lymphadenectomy.45 The most important recent evidence in support of radical lymphadenectomy comes from the Worldwide Esophageal Cancer Collaboration, who reported on their multi-institution international database comprising over 4600 resections for oesophageal cancer in patients who had not had induction therapy.46 They showed that prognosis was highly dependent on the number of lymph nodes involved; patients with more than three nodes involved had a 50% likelihood of systemic disease and patients with more than eight nodes involved had almost 100% likelihood of systemic disease.30,47 Even more important was their finding that survival depended not only on how many nodes were involved, but also on how many were removed at resection.47 The number of lymph nodes removed was the third strongest predictor of survival after depth of invasion and number of nodes involved. This finding has been corroborated in an analysis of the SEER (Surveillance, Epidemiology and End Results) database, both for oesophageal cancer48 and gastric cancer.49 It does appear from these data that the number of nodes resected has an effect on survival. Harvested nodal counts of 2347 and 3048 have been suggested as the optimal number, with caveats given that this is a goal and not an expected target in all patients. The recommendation for adequate staging for TNM7 is a minimum of six nodes. The AJCC (American Joint Committee on Cancer Staging) suggest 18 lymph nodes as the minimum number for accurate staging.50 The authors’ view is that this is too low. The role of the more extensive three-field dissection in oesophageal malignancy is less clear. Five-year survival rates showed no significant difference between two-field and three-field dissection for lower-third squamous tumours;32 the same pertains to patients with adenocarcinoma of the lower oesophagus. Patients with cancer of the upper thoracic oesophagus (third field) may benefit from dissection in the neck.24,38,51 There is little justification for oesophagectomy to be performed with intent to cure without any attempt to clear the first level of lymph nodes. Patients with either squamous carcinoma or adenocarcinoma of the oesophagus affecting the upper, middle and lower regions have mediastinal lymph node metastases in over 70% of cases.21,31,32,52 Over three-quarters of patients presenting with lower-third tumours have positive upper abdominal lymph nodes.53 In order to perform a potentially curative resection for carcinoma in the middle and lower thirds, a dissection of abdominal and mediastinal lymph nodes is therefore essential. After resection of the cervical, thoracic or abdominal oesophagus, one of three main paths can be used for reconstruction: presternal, retrosternal and posterior mediastinal (Fig. 5.4). Presternal route: This route is mentioned for historic completeness. It is approximately 2 cm longer than the retrosternal route, which in turn is approximately 2 cm longer than the posterior mediastinal route. The only indication for using this route is in the situation of multiple previous reconstructions that have compromised the other two routes. Retrosternal route (anterior mediastinal): The potential space between the sternum and the anterior mediastinum is easily opened up through effective dissection. There is reported to be a lower incidence of cervical anastomotic dehiscence compared with that of the presternal route. Its major disadvantage stems from the unnatural position of the cervical oesophagus in front of the trachea, which can result in an unpleasant sensation on swallowing. This route is used for reconstruction following emergency treatment of anastomotic dehiscence or the dehiscence of a gastric substitute that has caused posterior mediastinal sepsis. After incomplete resection (R1 and R2) there is some evidence that a retrosternal conduit would be preferable to the posterior mediastinal route.54 Reconstruction with stomach: The method of reconstruction should be kept as simple as possible, to minimise complications. The oesophageal replacement is determined by the site of the primary lesion. The stomach is the preferred option as this organ is easy to prepare and involves only one anastomosis. 1. The use of isoperistaltic stomach maintaining vascular supply. The right gastroepiploic and the right gastric artery and veins are vital to the viability of the stomach when used as an oesophageal substitute. The greater omentum is opened and the entire course of the right gastroepiploic artery is carefully identified and preserved. The vascular arcade is interrupted at the junction where the right gastroepiploic artery meets the left. The short gastric vessels are divided and ligated (Fig. 5.5). Figure 5.5 Main arteries of the stomach and points of division of vessels and stomach for oesophageal substitution. 2. Excision of the lesser curvature. Cancers of the lower two-thirds of the oesophagus require clearance of the lesser curve lymph nodes as well as the left gastric, common hepatic and proximal splenic artery lymph nodes. The left gastric artery should be ligated at its origin and resection of the proximal half of the lesser curvature of the stomach, including the cardia, is performed. The right gastric artery contributes to the maintenance of the gastric intramural vascular network and should be preserved if possible. Although the width of gastric conduit appeared not to impact on outcome in one study,56 the authors recommend using a gastric tube of 5 cm width or greater to minimise the risk of ischaemia as described by Akiyama et al.57 in the 1970s. 3. Preservation of the intramural vascular arcade. Extensive intramural arterial anastomoses between the vascular arcades of the lesser and greater curvatures exist. This has been well demonstrated by el-Eishi et al.58 and Thomas et al.59 This vascular network must be preserved during resection of the left gastric area of the lesser curvature and the cardia of the stomach. The extent of the resection of the lesser curvature is determined by a line connecting the highest point of the fundus (Fig. 5.6) and the lesser curvature at the junction of the right and left gastric arteries. This allows the removal of all potentially involved lymph nodes, yet preserves the arterial network to the fundus. There is no evidence that the trunk and descending branches of the left gastric artery running along the lesser curve need to be preserved and, from an oncological point of view, it is important that these are excised with the specimen. Care should be taken to ligate the short gastric vessels away from the greater curvature of the stomach to avoid damage to the intramural network and to preserve the extramural vascular network as well. The right gastroepiploic artery provides an adequate blood flow to maintain vascularity in the region of the fundus, which is the area used for anastomosis. 4. The high point of the stomach. The stomach is a flexible and capacious organ; its high point is the logical and sensible place at which to fashion an anastomosis with the remaining oesophagus. It is easily identified by applying traction with the surgeon’s fingers in an upward direction after all preparations have been completed. The stomach is transected as described previously (Fig. 5.6). 5. Gastric drainage. Pyloroplasty or pyloromyotomy after gastric reconstruction is contentious. There is some evidence that pyloroplasty reduces the incidence of gastric outlet obstruction.60 As short-term complications of pyloroplasty are minimal, the authors routinely perform a pyloroplasty to prevent the life-threatening early complications of gastric stasis and aspiration as well as late vomiting and bloatedness.46,61 1. Kocher manoeuvre. This manoeuvre allows the distance between the first part of the duodenum and the hiatus to be reduced. 2. Excision of the lesser curve of the stomach. When the lesser curve of the stomach is unusually short, an increase in length of the gastric substitute can be obtained, by dividing the lesser curve between curved clamps, before its resection. If absolutely necessary, a tense right gastric artery may be sacrificed by division at the level of the pylorus. 3. Incision of the serosa on the gastric wall. Multiple incisions placed in the gastric serosa may lengthen the stomach. A longitudinal incision placed along the resection line allows this to occur. The indications for this procedure are extremely rare. Reconstruction with colon: The principal indication for the use of colonic interposition is for tumours requiring an extensive oesophageal as well as gastric resection, although with thorough staging few of these patients are suitable for resection. A small proportion of patients presenting with oesophageal malignancy will have had a previous gastric resection for peptic ulcer disease, precluding the use of stomach as the oesophageal substitute. The numbers of patients such as this are diminishing. The choice of an oesophageal replacement under these circumstances lies between colon and jejunum. The colon is often recommended because of its advantage in having a greater capacity as a reservoir than the jejunum. Indications for colonic reconstruction (Box 5.3): It is preferable to use the colon in an isoperistaltic fashion. Unfortunately the vascular pattern of the colon varies and careful selection of the correct vascular pedicle to ensure viability of the transverse colon is essential. Each case requires evaluation on its own merit because of variations in anatomy. Not infrequently, the marginal artery is found to be of insufficient calibre to maintain viability of the transposed colon. Although the vascular appearance determines the appropriate colonic segment for use in each individual, the two possibilities for effective use of isoperistaltic colon are: (a) transverse colon based on the left colic vessels; (b) right colon based on the middle colic vessels. The disadvantage of transverse colon is that an abnormally narrow marginal artery may exist at the splenic flexure, compromising the blood supply of the proximal colonic segment. Preoperative assessment by angiography of the colonic vascular pathway has been suggested,62 but careful intraoperative observation of the vascular anatomy with temporary occlusion of vessels before division is a simple manoeuvre that is effective in most cases. Surgical technique: Preoperative mechanical bowel preparation is necessary, as is oral antibiotic cover to sterilise the bowel for 48 hours prior to surgery. The omentum is freed from the transverse colon and the hepatic and splenic flexures, while the entire colon is mobilised so that it can be placed outside the abdominal cavity for inspection of its vascular blood supply. Mobilising the sigmoid colon provides additional length so that the transverse colon can be tunnelled into the chest, to reach the neck. The proximal colon should be divided and, after anastomosis to the oesophagus, placed on sufficient stretch to prevent redundancy within the chest or in the substernal area. The colon should then be anchored in the straightened position by sutures to the crural margin of the hiatus, although not circumferentially. Continuity of the large bowel is re-established by end-to-end anastomosis, which is conveniently performed before the colo-jejunostomy or colo-gastrostomy for anatomical reasons. An excellent technical description of the use of various segments of colon has been provided by DeMeester et al.63 O’Sullivan and colleagues have described a useful refinement whereby resection of a short length of colon at either end of the chosen graft, leaving redundant mesentery, maximises blood supply at the critical points of the graft.64
Surgery for cancer of the oesophagus
Introduction
Surgical pathology
Surgical anatomy
Blood supply and lymphatic drainage
Preoperative surgical preparation
Nutritional support
Preoperative nutritional support
Respiratory care
Surgical objectives
Principles of oesophagectomy
Rules on resection margins
Resection of lymph nodes
Nodal tiers
The rationale for lymphadenectomy
Summary
Method of reconstruction of the oesophagus
Organ of reconstruction
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree