Fig. 9.1
Representative images in the evaluation of mechanical failure. These demonstrate: normal reservoir contour (a), early mechanical failure with deformation of the abdominal reservoir (b), late mechanical failure with loss of all contrast from the abdominal reservoir (c)
By comparison, non-mechanical failure, such as improper cuff sizing, urethral atrophy or erosion, is further evaluated with office cystoscopy, including device cycling/manipulation. Overall, urethral atrophy is the most common cause for revision surgery of AUS devices [6, 7]. Urethral atrophy is diagnosed when there is adequate fluid in the system, yet incomplete mucosal coaptation with device cycling during cystoscopy (Fig. 9.2). Likewise, ischemic changes of the urethral tissue underlying the cuff, such as tissue blanching, may be identified. During cystoscopic evaluation for urethral atrophy it important to note the position of the current urethral cuff and health of the surrounding urethral tissues as this may impact surgical planning. Similar to urethral atrophy, cystoscopic examination may identify urethral erosion, with visualization of the urethral cuff (Fig. 9.3).
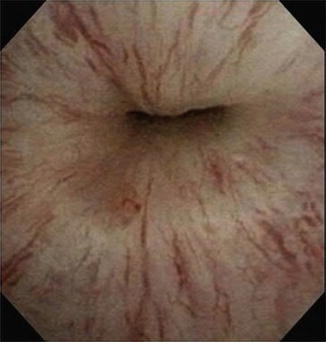
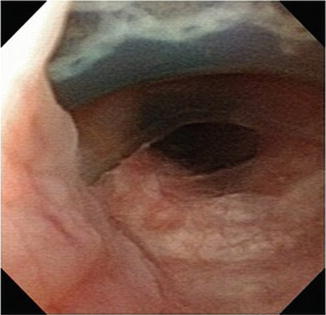

Fig. 9.2
Cystoscopy demonstrating incomplete urethral mucosal coaptation secondary to urethral atrophy

Fig. 9.3
Cystoscopy demonstrating a 180° dorsal urethral erosion
In addition to consideration of the above factors, evaluation of recurrent/persistent incontinence following male urethral sling placement includes further evaluation of sling and bladder function. For instance, sling migration/slippage, from lack of fixation or suture failure, may lead to recurrent/persistent incontinence. This can be evaluated with a repositioning test during office cystoscopy, which may help guide further management [8, 9]. Additionally, if not appreciated preoperatively, impaired bladder compliance or detrusor underactivity may also predispose to sling failure [8]. Thus, consideration can be given to performing a urodynamic evaluation if incontinence persists/recurs after urethral sling placement.
Anti-incontinence Surgery Following a Failed Artificial Urinary Sphincter
While the AUS is the gold standard for surgical management of severe male stress urinary incontinence, with 5 and 10-year device survival rates of roughly 75 % and 65 %, it is prone to failure over time [1, 7, 10, 11]. Of note, understanding the underlying cause of failure is crucial to optimal patient management. Here, we will focus on specific causes for recurrent or persistent SUI following AUS placement and their surgical management. Notably, the mainstay of surgical therapy following a failed AUS, is a repeat AUS as there is limited evidence regarding the efficacy of urethral sling placement in this setting [12, 13]. This is thought to be secondary to periurethral scarring following AUS placement which decreases the ability of the urethral sling to achieve adequate coaptation [12, 14].
Urethral Atrophy
The AUS achieves urinary continence by applying circumferential compression of the spongy tissues surrounding the urethra. While this mechanism of action allows for excellent functional outcomes, even in cases of severe leakage, this chronic pressure can compromise the underlying urethral tissue health and lead to urethral atrophy. In fact, urethral atrophy is the most common cause for a non-mechanical failure or device revision [6, 7, 15]. Typically, patients with urethral atrophy will report a gradual recurrence of stress urinary incontinence. The diagnosis is confirmed during cystoscopy, where incomplete urethral coaptation is visualized with device cycling (with adequate fluid in the system).
The treatment of AUS failure secondary to urethral atrophy is centered on device revision, though other strategies have been reported. Surgical options for AUS revision in cases of urethral atrophy include changing the location of the urethral cuff (moving proximally or distally), downsizing the urethral cuff, placement of a tandem urethral cuff or revising the pressure-regulating balloon. The decision between these management options is based on the local tissue quality, location of the in situ urethral cuff and surgeon preference.
Initial management of submucosal urethral atrophy is typically with downsizing the urethral cuff [15–17]. An advantage to urethral cuff downsizing is that no additional periurethral dissection is necessary. Of note, while previously downsizing could only occur to a urethral cuff size of 4.0, more recently the 3.5 cm urethral cuff has been introduced. Early reports with this smaller cuff size have been encouraging, though long-term data is lacking [18]. Additionally, an increased rate of erosion has been reported in patients treated with this smaller cuff in the setting of prior pelvic radiation, compared to those without this exposure (21 % versus 4 %) [19].
In cases where the smallest available cuff is already in situ or cuff downsizing has previously failed, other options include: relocation of the urethral cuff, placement of tandem urethral cuffs, or manipulation of the pressure-regulating balloon. Changing the location of the urethral cuff is an excellent option when an area of healthy urethra can be identified in a more proximal or slightly more distal location that would allow for adequate tissue coaptation. Of note, as the caliber of the urethra tapers distally, careful attention must be given to appropriate cuff sizing in order to avoid persistent SUI. In order to account for urethral tapering distally, use of a transcorporal technique for added tissue bulk in the setting of revision for urethral atrophy has been described [20].
Another management strategy for submucosal urethral atrophy is placement of a tandem urethral cuff. Tandem urethral cuff placement attempts to avoid increasing the pressure on the atrophic urethra segment and instead distributes additional compression to a second area of the urethra [21]. With regard to the technique of tandem urethral cuff placement, circumferential urethral dissection roughly 2 cm distal to the primary cuff is needed (Fig. 9.4a). In cases with difficult tissue planes, a transcorporal approach to tandem cuff placement can be utilized [22]. The device tubing emanating from the existing cuff is dissected free, controlled with rubber shods and transected. Following this the new cuff is attached with the use of a Y-shaped adapter and Prolene free-ties (Fig. 9.4b). We prefer to use this approach, rather than additional Quick-Connect attachments in the perineum, as these are more bulky and have in some cases have led to cutaneous erosions. Notably, given the additional urethral cuff, we typically add 3 cc of fluid (normal saline or contrast) to the system. Notably, excellent surgical outcomes have been reported with a tandem cuff technique [21, 23]. However, compared to single cuff placement, tandem cuff placement may have higher rate of urinary retention and revision surgery [24]. Additionally, higher rates of erosion have been associated with distal urethral cuff placement [25].
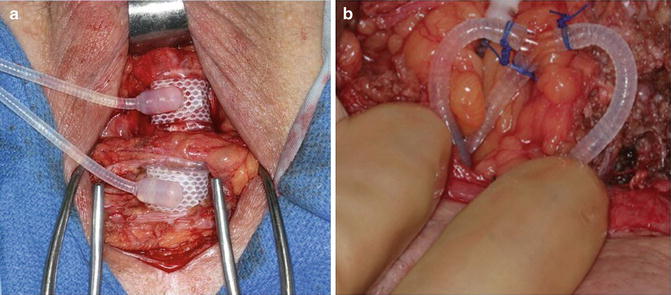

Fig. 9.4
Tandem urethral cuff placement (a), connection of tandem urethral cuff to in situ system (b)
An additional option for AUS revision for recurrent incontinence secondary to urethral atrophy is exchange of the pressure-regulating balloon. It is hypothesized that this may be successful due to laxity in the reservoir, which may develop over time [6]. One advantage to such an approach is avoidance of a repeat urethral dissection, which may risk urethral injury and device infection. Replacement of the reservoir with a higher-pressure reservoir is not recommended as this may increase the risk of erosion [15].
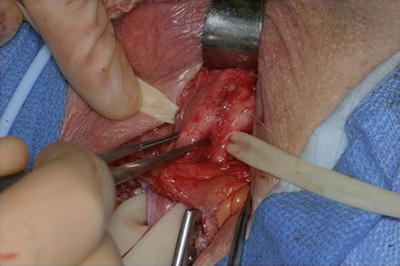
Several further adjunctive techniques have also been described in the management of recurrent SUI secondary to urethral atrophy in patients with multiple prior AUS failures. One such strategy is increasing the underlying urethral tissue bulk by placement of a small intestinal submucosal urethral wrap [26, 27] (Fig. 9.5). In two small series adequate outcomes were obtained, though patients remained at higher risk for erosion and device failure [26, 27]. Additionally, in a small series, placement of a urethral sling proximally for additional compression in cases of urethral atrophy from an AUS has been reported [13].
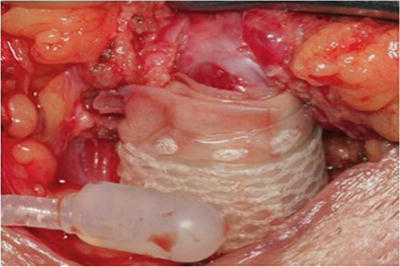

Fig. 9.5
Intraoperative image of urethral bulking with submucosal intestinal wrap prior to AUS cuff placement
Mechanical Failure
As with any prosthetic device, recurrent incontinence secondary to device malfunction may occur over time. In one series, revision for malfunction cases accounted for 25 % of revision surgeries [15]. Device malfunction can be identified on physical exam with improper device cycling and lack of coaptation of the urethral mucosa during cystoscopy. In addition, imaging with either an abdominal X-ray (if contrast was used to fill the system) or ultrasound (if normal saline was used) will confirm the diagnosis [4, 5].
Surgical management in these cases is dependent on the timing of the initial AUS placement. While no universal consensus exists, most reports suggest that when undergoing surgery for device malfunction, removal and replacement of an isolated component may be considered within 3 years of the initial implantation [6, 16]. If the device has been in situ longer than 3 years, or there is concern about other component function, removal and replacement of all components can be considered. Evaluation of additional components can be performed with intraoperative fluoroscopy and contrast instillation, use of a pressure transducer or by aspirating and measuring the fluid in the balloon [28]. Previous testing of methods for evaluating hydraulic leakage demonstrated inaccuracies with volume, pressure or electric conductance measurements for AUS devices and recommended visual evaluation of the components. Notably, this series did not evaluate the use of contrast in the device and intraoperative fluoroscopy, which is our preferred method.
A few technical considerations during AUS revision for malfunction are worth noting. First, we typically begin these cases with the perineal dissection and evaluation of the indwelling urethral cuff. This is performed first as the urethral cuff is the most commonly reported site of device malfunction [7, 16]. Dissection is carried directly on to the urethral cuff in order to exposure the cuff in its entirety and the perineal portion of the tubing. The urethral cuff is removed and tested with saline injection. The periurethral dissection is preserved with a Penrose drain which is placed around the urethral. If a leak is found from the urethral cuff, this component is replaced and reconnected to the in situ pump and reservoir via the Quick-Connect fastener (Fig. 9.6a). If no leak is detected the cuff is removed and dissection proceeds with evaluation of the abdominal reservoir and pump (Fig. 9.6b). When the abdominal components are evaluated, we tend to replace the entire device as the dissection has already been completed. One important technical consideration is ensuring the replacement of fluid in the system when replacing a single component. In these cases, we determine the volume that needs to be added based on the preoperative X-ray (presence/absence of residual contrast) and confirm with intraoperative imaging.
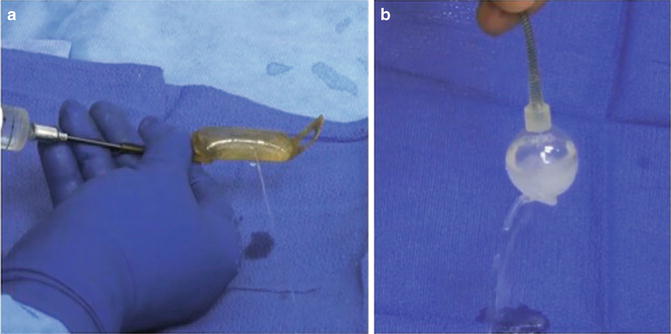

Fig. 9.6
Intraoperative image of device malfunction secondary to urethral cuff leak (a), abdominal reservoir leak (b)
With regard to outcomes for revision for either mechanical failure or urethral atrophy, several series have found results comparable to primary AUS implantation [15, 29]. In the series by Raj et al. 5-year device durability was demonstrated in 80 % of primary and 88 % of secondary AUS implantations [15]. Likewise, excellent function outcomes were demonstrated, with 90 % of primary and 82 % of secondary AUS placements using zero to one pad [15].
Urethral Erosion/Device Infection
One of the most worrisome complications of AUS placement is device infection/erosion, which has been reported in 0.46–9.5 % of primary implantations [7, 30, 31]. Typically, these entities are identified through a combination of history and physical exam demonstrating signs or symptoms concerning for underlying abscess/infection, and/or evidence of urethral erosion on cystoscopy.
Device infection and erosion may be encountered from unrecognized intraoperative injury or contamination, postoperative wound infection, compromised urethral tissues, hematogenous spread of bacteria during non-genitourinary procedures or other patient related factors. In one report, the most common organisms isolated at time of device explantation for infection were Staphylococcus aureus, Staphylococcus epidermidis, and gram-negative bacilli [32]. In these settings, patients should undergo reoperation with explantation of all device components, as they are considered infected/contaminated. In cases of urethral erosion, management of the site of urethral erosion has typically been via indwelling urethral Foley catheter, though recently ventral urethroplasty at the time of explantation, in an attempt to decrease stricture formation, has been reported [10, 33–35]. Following acute management, the catheter is left in place for several weeks to allow for adequate healing. In these cases, we prefer 6 weeks of postoperative catheterization with peri-catheter retrograde urethral imaging performed prior to catheter removal, to rule out persistent urethral extravasation. Notably, immediate AUS replacement following removal of an infection, non-eroded, AUS has been reported, but is not considered standard practice [36].
Following repeat evaluation 4–6 months after device explantation for infection or erosion, salvage AUS implantation may be considered. This evaluation should include history, physical examination, urinalysis, post-void residual and cystoscopy to rule out urethral stricture. If adequate urethral healing has occurred and the patient wishes to proceed, reimplantation can be considered. Notably, these are difficult reoperative cases secondary to scarring and loss of tissue planes from the previous inflammation and infection. With repeat perineal dissection we attempt to avoid excessive mobilization of the urethra in an effort to preserve already tenuous blood supply. Depending on the tissue quality, an adequate location for cuff placement is determined. In many of these cases, a transcorporal approach is needed secondary to compromised urethral tissue or obliterated dissection planes [10, 20, 22] (Fig. 9.7). Notably, preservation of erectile function with use of the transcorporal approach has been reported [37]. Lastly, as with all AUS placements, evaluation of urethral integrity following periurethral dissection (either via cystoscopy or pericatheter fluid injection) is needed.