9
Radiotherapy and chemotherapy in treatment of oesophageal and gastric cancer
Introduction
The identification of improved activity when chemotherapy and radiotherapy are given synchronously has already led to chemoradiotherapy (CRT) becoming the primary organ-preserving approach in anal, cervix and certain head and neck cancers, with surgery being reserved for salvage.1,2 There is now good evidence that primary CRT has a role in oesophageal cancer treatment.
Oesophageal cancer
Potentially curative treatment
Theoretical and generic issues of preoperative versus postoperative therapy treatment include:
• a more easily defined target volume;
• improved tumour oxygenation at the time of treatment;
• the potential to improve resectability and reduce the impact of tumour cell spillage at surgery;
• improved chance of an R0 resection and thereby the reduction of local recurrence;
• improved chance of treating micrometastatic disease;
• patient likely to be better able to tolerate adjuvant therapy prior to major surgery than following it;
• may improve swallowing and therefore nutrition prior to surgery;
• spare those patients that progress early with metastatic disease major surgery.
• the overtreatment of some patients that will not benefit from non-targeted therapy as opposed to targeting patients with factors that may determine the likely risk and site of residual disease;
• may make patient less well physiologically prior to major surgery, increasing risk of perioperative morbidity and mortality;
• may allow disease progression prior to definitive treatment.
Preoperative radiotherapy alone
This approach has been shown to be of value in rectal cancer.3 There have been six randomised trials of preoperative radiotherapy. Three trials were restricted to squamous carcinoma. One of these, by Gignoux et al., reported an improvement in local/regional recurrence (46% vs. 67%).4 Nygaard et al. report improved survival, but this series is complicated by the inclusion of some patients also receiving chemotherapy.5 One trial included both squamous and adenocarcinoma,6 and two do not specify the histology. Overall it is difficult to draw firm conclusions from these trials.
Postoperative radiotherapy
There are four randomised trials in the literature. The numbers are small (totalling 843 adjuvant patients), and three out of the four include only squamous carcinoma. Teniere et al.8 showed no survival advantage in 221 patients. There was a small improvement in the failure rate but at the cost of significant side-effects. The benefit appears to be limited to node-negative patients. Fok et al.9 included both adenocarcinoma and squamous carcinoma. Whilst both curative and palliative resections were included, the patients were separately analysed and received different radiotherapy doses. The results show a significant morbidity (37%) and mortality related to bleeding from the transposed intrathoracic stomach. It should be noted that the dose per fraction of the radiotherapy was high (3.5 Gy), which may be significant. There was a lower intrathoracic recurrence rate, particularly relating to tracheobronchial disease.
A larger randomised study from China included 495 well-staged patients with squamous carcinoma randomised to receive either surgery alone (S) or surgery and postoperative radiotherapy (S + R).10 Whilst there are significant concerns about the ethics (the patients were not aware they were in a trial and so did not give appropriate consent), the study was still published because of its significant results. The surgery appears to be of a high standard and included a radical lymph node dissection. The radiotherapy was wide field and included the bilateral supraclavicular fossae (SCF), mediastinum and anastomosis to an initial dose of 40 Gy. A further 10 Gy was given to the SCF and 20 Gy to the mediastinum by a different technique, allowing a maximum dose to the transposed stomach of 50 Gy. There was a relatively high proportion of earlier stage IIA disease in the study compared with a UK population. The analysis showed a highly significant difference in 1-, 3- and 5-year survival in stage III disease between the S and S + R arms (67.5%, 23.3%, 13.1% vs. 75.5%, 43.2%, 35.1%, respectively). The pattern of relapse was different between the two arms, with significantly fewer recurrences in the neck, SCF and mediastinum. Unlike other studies, toxicity to the transposed stomach was minimal.
The role of postoperative radiotherapy-based treatment in the case of a histological R1 resection is even less clear. There have been no randomised trials addressing this group of patients; indeed, the quality of reporting of circumferential resection margin (CRM) involvement by microscopic disease, which is influenced by postoperative surgical dissection of the operative specimen, is variable. In the absence of randomised evidence, the knowledge that radiotherapy has a proven role in oesophageal cancer probably justifies considering patients with longitudinal resection margin involvement for postoperative radiotherapy on an individual patient basis. When undertaken, there is some evidence that one should attempt to encompass both the anastamosis and the tumour bed but in the case of a high anastomosis for a lower oesophageal cancer, which is difficult to see radiologically, this can be challenging and requires specialised multidisciplinary input. The role for radiotherapy treatment in the case of CRM involvement is unclear, but it would seem sensible to target those patients where the risk of systemic disease relapse is lower, i.e. those with a lower ratio of involved lymph nodes.11
Preoperative chemotherapy
Preoperative chemotherapy in both squamous and adenocarcinoma appears to achieve consistently good clinical response rates, ranging from 47% to 61%.12,13 Early studies, predominantly in squamous carcinoma, used combinations of cisplatin, vindesine and bleomycin. More recently, cisplatin and 5-fluorouracil (5-FU) combinations have been used in important randomised trials. New 5-hydroxytryptamine-3 (5-HT3) antagonist antiemetic drugs have allowed cisplatin to be used with dramatically reduced toxicity. Protracted venous infusion (PVI) of 5-FU, and more recently capecitabine, an oral 5-FU prodrug, in combination with cisplatin and epirubicin (the ECF regimen) has produced increased response rates in non-randomised studies. These more modern cisplatin–5-FU combinations seem to be active in both squamous14 and adenocarcinoma,13 although the benefit of anthracycline therapy, i.e. epirubicin, in squamous cell carcinoma is less certain and is therefore often omitted.
Randomised trials of preoperative chemotherapy
The American Intergroup Trial (INT 0113) produced data on 440 randomised patients with a median follow-up of 46.5 months.15 Adenocarcinoma (54%) was the predominant histology. The chemotherapy given was three preoperative courses (cisplatin and 5 days of infusional 5-FU) and in stable or responding patients two postoperative courses. Overall, 83% of patients received the intended two preoperative cycles of chemotherapy. However, only 32% of patients received both postoperative chemotherapy cycles. There was no difference in treatment-related mortality between the two arms (6% surgery (S) vs. 7% chemotherapy (C) + surgery (S); P = 0.33). On an intent-to-treat basis there was no difference in median survival (16.1 months C + S vs. 14.9 months S), and 1-, 2- and 3-year (23% C + S vs. 26% S) survivals. Disappointingly, there was no difference in the pattern of metastatic disease between the two arms. However, there was a significantly higher rate of R1 resections in the surgery-alone arm.
The Medical Research Council (MRC) OEO2 study is the largest and arguably the most influential trial in this area.16 A total of 802 patients were randomised to receive two courses of cisplatin and a 4-day infusion of 5-FU followed by surgery (CS) after 3–5 weeks or immediate surgery alone (S) and showed a significant survival advantage for patients receiving preoperative chemotherapy.
The overall survival rate was significantly improved with preoperative chemotherapy (P = 0.004; hazard ratio 0.79, CI 0.67–0.93), with an estimated reduction in risk of death of 21% and 2-year survival figures of 43% CS vs. 34% S. There was no evidence that the effect of chemotherapy varied with histology. Long-term follow-up with a median follow-up of 6 years has confirmed these results, with 5-year survivals of 23% CS vs. 17% S.17
The recently completed MRC/NCRI trial in the UK (OEO5) compared OEO2 chemotherapy with four cycles of ECX (epirubicin–cisplatin–capecitabine) in adenocarcinoma alone. The high completion rate and positive results of preoperative chemotherapy in the MRC MAGIC (ST02) study19 for gastric and gastro-oesophageal cancer pointed to the strategy of using a modified ECF regimen, which is accepted in the UK as the best standard of care for advanced gastro-oesophageal cancer, and using it in a neoadjuvant setting to try and improve on the results of OEO2. The results of the REAL2 study,20 a phase III trial of palliative chemotherapy, showed that the oral fluoropyrimidine (capecitabine) could be substituted for infusional 5-FU with safety and at least equivalent efficacy. The advantage of easier chemotherapy delivery without the use of Hickman lines and their associated morbidity is a step forward. This study is also important in that it places an emphasis on high-quality assurance of staging, surgery, chemotherapy and pathology. There is little doubt that at least one of the reasons for differing results in trials in the whole area of gastro-oesophageal cancer has been a wide variation in the quality of staging modalities and surgery, as well as the heterogeneity in the regimens tested and trial design. The MRC OEO5 trial attempted to set high standards that should translate into improved patient selection and outcomes, even within the control arm.
Postoperative chemotherapy
There are few useful trials that address the question of adjuvant postoperative chemotherapy. The trials reported by Roth et al.21 and Kelsen et al.15 both have an adjuvant component, coupled with preoperative treatment. The fact that only 32% completed the postoperative phase in the Intergroup study underlines a problem with this approach.15 Patients undergoing major resections for oesophageal carcinoma often have a prolonged postoperative phase. The start of chemotherapy may be delayed due to performance status. Patients may also choose not to continue. A strategy that relies solely on postoperative treatment may have significant problems. Improved patient selection and postoperative supportive care may allow this approach to be practical. The MAGIC gastric cancer trial latterly included tumours of the gastro-oesophageal junction and lower oesophagus and intended three postoperative courses of ECF as well as three given preoperatively in the protocol. Again, only 40% completed the postoperative chemotherapy. The trial has shown an improvement in overall survival, as described in the section on gastric cancer,19 which lends further support for the concept of neoadjuvant chemotherapy for cancers of the oesophagus or gastro-oesophageal junction.
Preoperative chemoradiotherapy
Non-randomised studies of CRT have appeared in the literature since the late 1980s. The review article by Geh et al.22 summarises 46 trials containing 20 patients or more. Overall, pooled data from these studies show that, of 2704 patients (squamous 68% and adenocarcinoma 32%), 79% were operated on with a pCR rate of 24% of those treated and 32% of those resected. As experience with this modality of treatment has grown, lessons have been learned. Attempts to escalate the dose of radiotherapy can lead to unacceptable rates of morbidity, especially if higher doses per fraction are used.23,24 Reported CRT-related deaths in the non-randomised series ranged from 0% to 15% (mean 3%). Postoperative deaths ranged from 0% to 29% (mean 9%). Adult respiratory distress syndrome, anastomotic leak and breakdown, pneumonia and sepsis were the commonest causes of death following oesophageal resection. Treatment-related deaths ranged from 3% to 25% (mean 9%) of all patients treated. It seems clear that the risk of chemotherapy-related toxicity, particularly myelosuppression, rises with the number of drugs used and the intensity of the CRT regimen.25,26 An increased risk of tracheobronchial fistula has been reported.27 However, most of the reported series did not have the latest sophisticated radiotherapy techniques that allow greater precision and sparing of organs and tissues to within normal tissue tolerance.
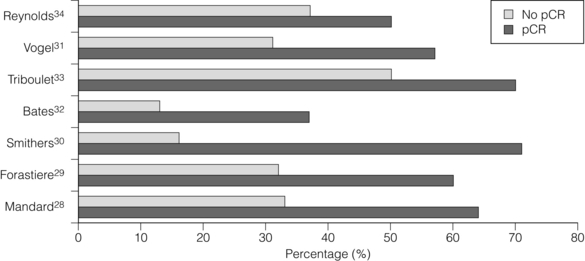
Consistent reporting of pathology is important, and a grading of CRT response has been described by Mandard et al.28 Five grades of response ranging from no identifiable tumour to complete absence of regression allow a more objective approach to be adopted. In this paper the significant predictor of disease-free survival after multivariate analysis was the tumour regression grade. There is evidence that pCR confers a survival advantage over those patients not achieving pCR.29–34 In Fig. 9.1, different comparative outcomes, such as median survival in months, overall or disease-free survival in years, are plotted together in the series, quoting outcomes separately. The importance is in the consistent nature of the difference in outcomes in each series. It becomes clear that prediction of this response prior to treatment either through molecular markers or PET activity after induction chemotherapy alone might allow very different algorithms of treatment modalities (also see Chapter 3).
Table 9.1 summarises nine reported randomised trials of preoperative CRT compared with surgery alone. In four of these the chemotherapy was given sequentially to the radiotherapy and in four synchronously. Two trials using sequential treatment in squamous carcinoma received relatively low doses of radiotherapy and showed no convincing evidence of improved survival with the combined treatment.6,35 In a larger European Organisation for Research and Treatment of Cancer (EORTC) trial involving 282 patients, the cisplatin chemotherapy was given in close sequence with the radiotherapy.24 The radiotherapy was given in a split course and at a relatively high dose per fraction (two courses of 18.5 Gy in five daily fractions split 2 weeks apart). The CRT patients were more likely to have a curative resection. The disease-free survival was significantly longer (3-year CRT + S 40% vs. S 28%). There was no difference in the overall survival, largely due to a significantly higher postoperative mortality in the CRT arm (12% vs. 4%). Apinop et al.36 reported a synchronous CRT series of 69 squamous histology patients with no improvement in survival.
Table 9.1
Randomised trials of preoperative chemoradiation
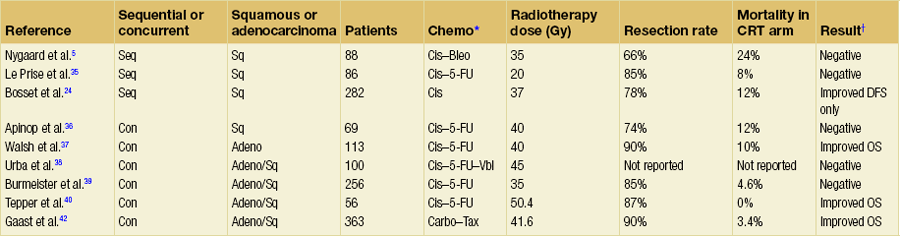
*Bleo, bleomycin; Carbo, carboplatin; Cis, cisplatin; 5-FU, 5-fluorouracil; Tax, paclitaxel; Vbl, vinblastine.
There are four larger trials of preoperative synchronous CRT.
The University of Michigan trial38 randomised 100 patients including both squamous and adenocarcinoma. The surgery was a transhiatal resection. Patients in the CRT arm received 45 Gy in 30 fractions with cisplatin, 5-FU and vinblastine. At first analysis there was no significant difference between the arms but at 3 years a statistically significant benefit to the combined treatment emerged, with overall survival of 32% vs. 15%. A final analysis has shown no survival advantage and demonstrates the danger of early publication of a trial that was essentially underpowered.
The results of the Australasian Gastro-Intestinal Trials Group (AGITG)39 have been criticised for having a low radiotherapy dose and only one cycle of cisplatin and 5-FU chemotherapy. Although the trial was negative overall there are some clues for the direction of future approaches. There was a significant survival difference in patients with squamous histology (36% of the total) with the addition of CRT and a much higher pathological complete response rate.
The US trial NCCTG-C9781 (CALGB 9781) closed prematurely as a result of poor recruitment due to a reluctance to recruit patients to a trial with a no treatment arm. However, mature results from CALGB 9781 are available and despite small numbers show a significant improvement in overall survival in preoperative CRT compared to surgery alone (5-year survival of 39% vs. 16%).40 Resection rates were high in the preoperative CRT arm (87%) and there was no increase in operative mortality. The trial included higher quality staging and surgery.
Interpretation of such heterogeneous trials, in the regimen being tested, design and outcomes, is difficult. Nevertheless, a meta-analysis of randomised trials has shown that this approach increases R0 resection rates, reduces locoregional recurrence and improves survival compared with surgery alone.41 More recently, and not included in the meta-analysis above, a randomised phase III study comparing surgery alone to preoperative CRT has shown a near doubling of overall survival (OS) in favour of the preoperative arm (OS 49 vs. 26 months, hazard ratio (HR) 0.67), a pCR rate of 32% and no increase in surgical mortality (3.8% (S) vs. 3.4% (CRT-S)).42 In the ‘CROSS’ trial, 363 patients with operable oesophageal or gastro-oesophageal junction tumours were randomised to surgery alone or to a preoperative CRT regimen of weekly carboplatin (AUC2) and paclitaxel (50 mg/m2) concurrent with radiotherapy (41.4 Gy in 23 fractions). Of the 175 patients assigned to the CRT arm, 163 completed protocol treatment and the study reported a low incidence of grade 3/4 CRT toxicity (haematological, 6.8%; non-haematological, 16%). The R0 resection rates in the surgery and CRT + surgery arms were 67% and 92.3%, respectively (P = 0.002). The results of this study, performed in patients with a similar stage and morphological distribution to those in the UK, would suggest that where preoperative CRT is delivered safely, this may lead to a significant improvement in outcome.
Neoadjuvant chemoradiotherapy or chemotherapy?
There is rightly a clear separation in future trials for adenocarcinoma and squamous carcinoma. As the trend moves towards squamous cancers being treated with primary CRT, the role of preoperative CRT may be revisited as a means of improving the outcome for patients with adenocarcinoma. The majority of such patients will present with stage III disease (at least T3 with lymph node metastases). Such tumours frequently threaten the circumferential margin of surgical resection (CRM), although a clear plane for surgical excision does not exist as it does for other anatomical sites such as the rectum. Disease present at or within 1 mm of the circumferential margin (R1) occurs in more than 50% of stage III cases11,44 and is a poor prognostic factor. In the OEO2 study, the 3-year and median survival for patients with R0 and R1 resection were reported as 42.4% vs. 18% and 2.1 years vs. 1.1 years, respectively.16 Preoperative chemoradiotherapy (CRT) has become a standard management strategy in rectal cancer for patients who have a threatened CRM on preoperative staging.
There has been only one randomised phase III trial comparing preoperative chemotherapy with preoperative CRT. This study by Stahl et al. aimed to recruit 354 patients to detect a 10% improvement in OS in favour of CRT (from 25% to 35%) but closed early as only 126 patients could be recruited in 5 years. Nonetheless, it showed a non-significant trend towards improved 3-year survival in favour of CRT (47.4% vs. 27.7%, P = 0.07).45
The undoubted extra toxicity may be justified for this selected group and is infinitely preferable to postoperative treatment. New radiotherapy technology allows more accurate treatment delivery and lower morbidity, and when coupled with higher quality surgery and perioperative care should allow the sort of overall results from the Dutch trial42 to be reproduced. Whatever improvements in locoregional treatments are proposed, the highest risk to be faced and addressed with new trials for stage III adenocarcinoma is ultimate systemic relapse. Trials with new biological agents added to standard chemotherapy or selective CRT are likely to be the next step, with advance knowledge from their use in the advanced and metastatic disease setting.
Definitive radiotherapy and chemoradiotherapy
Definitive radiotherapy
Classical figures quoted for survival from radical radiotherapy come from the paper from Earlam and Cunha-Melo.46 Mean survival figures of 8489 patients at 1, 2 and 5 years were 18%, 8% and 6%, respectively. Approximately 50% of patients were treated with curative intent. Older series tend to be of squamous carcinoma treated with radiotherapy alone. Modern radiotherapy in more selected patients can produce impressive survival results. In a series of 101 patients treated at the Christie Hospital in Manchester between 1985 and 1994, 3- and 5-year survival figures of 27% and 21%, respectively, were recorded.47 There was a slightly better survival for adenocarcinoma, but not reaching statistical significance. The majority of tumours (96/101) were of 5 cm or less in length. Importantly, the only significant prognostic factor was the use of diagnostic CT, introduced during the latter part of the study. This was used to plan the radiotherapy and led to an increase in field sizes. The conclusion of the paper was that radiotherapy provided an effective alternative to surgery and that modern radiotherapy planning techniques may improve results.
There is no reason to compromise on staging or treatment planning standards and with modern technology high doses can be given with low morbidity. A selected series of 51 patients 80 years and over with squamous carcinoma treated with 66 Gy of radiotherapy in Japan produced median survival of 30 months and a 3-year survival rate of 39%.48
Definitive chemoradiotherapy
The adoption of CRT stems from high response rates and in particular high pCR rates seen in patients going on to resection. There are four randomised trials comparing radiotherapy alone with CRT. Three of these use low doses or low intensity of chemotherapy. A small series of 59 patients from Brazil did not demonstrate a significant survival advantage.49 The response rates and 5-year survival rates (6% vs. 16%) were better in the CRT arm but at a cost of increased acute toxicity. An important non-randomised series is reported by Coia et al.50 Treatment was with infusional 5-FU and mitomycin C with 60 Gy of radiotherapy. Patients with early-stage disease are reported separately. The respective 5-year survival and local failure rates, in clinical stages I and II combined, were 30% and 25%. There was no treatment-related mortality, although there was increased acute toxicity (22% grade III and 6% grade IV).
The high local failure rate of 45% in the Herskovic trial led to the Intergroup study 00123 (Minsky) that compared a regimen similar to the Herskovic regimen (modified with narrower radiotherapy fields, radiotherapy using 1.8 Gy/fraction and an alteration in the chemotherapy schedule to reduce anticipated toxicity), to the same schedule but with a higher dose of radiotherapy (64.8 Gy in 36 fractions).52 In total, 236 patients, once again predominantly with squamous cell cancer, were randomised within this study. The trial had to be closed prematurely due to an excess of treatment-related deaths in the experimental arm (11 vs. 2), although the majority of these occurred before the higher dose section of the treatment protocol had been received. Although this trial did not show better disease control with higher doses of radiotherapy (56% failure at 2 years compared to 52% in the standard arm) it did demonstrate remarkably consistent outcomes of, approximately 30% survival at 3 years with definitive chemoradiotherapy.
Another approach to improve local control was to use brachytherapy to intensify the radiotherapy dose to the tumour. Study RTOG 92-07 used the 50-Gy external beam and chemotherapy protocol from the Herskovic protocol and added an intraluminal brachytherapy boost with one of two methods of delivery, high dose rate or low dose rate.53 Six of the 35 patients developed an oesophageal fistula and this toxicity was deemed unacceptable.
Following successful CRT or radiotherapy alone there is a significant rate of benign stricture formation. This ranges from 12%54 to 25%50 in more modern studies. However, good swallowing function can be maintained in the majority of patients. Even in those with a benign stricture, a full or soft diet can be maintained by dilations in 71% of cases.55 The treatment of post-CRT benign stricture with stents has not been successful in the authors’ experience and gives rise to mediastinal pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree