17
Paraoesophageal hernia and gastric volvulus
Introduction
Paraoesophageal hiatal hernia is a relatively rare condition comprising approximately 15% of hiatal hernias. This condition was first identified on post-mortem examination in 1903,1 and on upper gastrointestinal contrast radiography by Akerlund et al. in 1926.2 Since that time, the importance of these hernias has been recognised because of the resulting life-threatening complications including gastrointestinal bleeding, iron deficiency anaemia, gastric volvulus with subsequent strangulation and perforation.3 Management of paraoesophageal hernias has changed considerably since the development of laparoscopic repair and several areas of controversy remain as this technique continues to evolve.
Epidemiology
Hiatal hernias occur in approximately 10% of the population, with approximately 15% of these being paraoesophageal hernias.4 Risk factors for hiatal hernias include age greater than 50, body mass index greater than 25 kg/m2 and male gender.5 There is also a familial occurrence that confers a 20-fold increased risk in younger siblings of children with a hiatal hernia.6
Anatomy and natural history
The oesophagus enters the abdomen via the oesophageal hiatus of the diaphragm, which is comprised of the limbs of the right diaphragmatic crus,7 although varying degrees of contribution of the left crus are often present. Although not anatomically correct, descriptions of hiatal dissection and repair, including those presented here, typically refer to these limbs as the right and left diaphragmatic crura or pillars. The intra-abdominal oesophagus is anchored to the diaphragm by the phreno-oesophageal ligament, which maintains the position of the squamocolumnar junction within or slightly distal to the diaphragmatic hiatus and prevents displacement of the stomach through the diaphragm.8
Derangements in the normal anatomy of the gastro-oesophageal junction and oesophageal hiatus allow herniation of the stomach through this opening into the thoracic cavity. The aetiology of these hernias is often unclear. Hiatal hernias are rare in Asian and African populations, and are more common in conditions of increased intra-abdominal pressure, such as obesity and pregnancy. Hiatal hernias are classified as types I–IV, with types II–IV representing forms of paraoesophageal hernia (Fig. 17.1). This nomenclature is slightly confusing, as giant hiatal hernias may appear to be a sliding or paraoesophageal hiatal hernia depending on the patient position. We prefer to use the term ‘giant hiatal hernias’ for the large hernias that are usually classified as type III and IV paraoesophageal hernia.
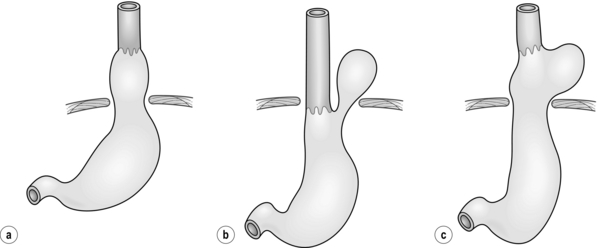
Figure 17.1 Classification of hiatal hernias. (a) Type I (sliding). (b) Type II (true paraoesophageal). (c) Type III (combined).
Most hiatal hernias (90%) are type I, or sliding, hernias in which the gastric cardia herniates upwards with proximal migration of the lower oesophageal sphincter into the thorax. The phreno-oesophageal ligament is attenuated, but remains intact.9 The term ‘sliding hiatal hernia’ is applied here because the gastric wall comprises a portion of the hernia sac, analogous to retroperitoneal structures in sliding inguinal hernias.
Type III (combined) hiatal hernias involve elements of both type I and II hernias, and represent the majority of paraoesophageal hernias presenting for surgical repair. These hernias result from enlargement of a type I hernia defect, to allow cephalad migration of the stomach in response to the transdiaphragmatic pressure gradient. There is a true hernia sac present with fundic herniation and proximal migration of the gastro-oesophageal junction into the thorax. This type of hernia is associated with laxity of the elements that retain normal gastric position, and the natural history is to progress to complete gastric herniation, with the appearance of an upside-down intrathoracic stomach on contrast radiography.10 This increased gastric mobility predisposes patients to gastric volvulus, which we will address in detail later in this chapter.
As with all hernias, the natural history of paraoesophageal hernia is progressive enlargement over time. Early in their course some are clinically silent, but most people have gastro-oesophageal reflux symptoms, which may have been treated with medical therapy or not at all. By the time the hernia grows large enough to allow a paraoesophageal hernia of the stomach, a cardio-oesophageal angle is often recreated, which may cause reflux symptoms to wane as the paraoesophageal herniation recreates a more competent antireflux valve.11 The term ‘giant paraoesophageal hiatal hernia’ refers to defects in which at least half of the stomach is located within the thorax on contrast radiography, the hernia measures at least 6 cm in length on preoperative endoscopy, or a distance between the crura of at least 5 cm is noted on intraoperative inspection.12,13 These hernias are repaired using the same principles required for all paraoesophageal hernias, but the large hernia sac and propensity for oesophageal shortening make these cases especially challenging.
Presentation and diagnosis
Approximately half of all paraoesophageal hernias are clinically silent and become apparent on imaging studies obtained for another reason. Symptomatic hernias may present with epigastric or chest pain, heartburn, postprandial fullness, regurgitation or dysphagia. Many of the signs and symptoms are non-specific and may mimic those of acute myocardial infarction, gastric ulcer or pneumonia. Type II hernias typically present without reflux symptoms, whereas type III hernias most typically present with postprandial chest pain with or without reflux symptoms (e.g. heartburn, dysphagia, regurgitation). Others present with iron deficiency anaemia secondary to chronic blood loss from erosions of the gastric mucosa caused by repeated movement across the hiatus, a phenomenon originally described by Collis in 1961,14 or from an ulcer at the level of the diaphragm, described by Cameron from the Mayo Clinic.15,16
Operative indications
It has been generally accepted that reasonable surgical candidates should undergo repair regardless of symptoms. This recommendation is based on early series that showed an increased mortality after emergency surgery of 30% compared to 1% in elective cases. In 1967, Skinner and Belsey found that 6 of 21 patients with a known diagnosis of paraoesophageal hernia died from complications of their hernia when followed conservatively for 5 years.12
More recent studies have suggested differences in both the natural history of the disease and operative outcomes. Allen et al. followed 23 patients who refused operative repair of paraoesophageal hernias for a median follow-up of 78 months without development of any life-threatening complications.17 Others have advocated that asymptomatic or minimally symptomatic paraoesophageal hernias may be managed by a strategy of ‘watchful waiting’, with emergency surgery required in only 1.2% of cases and an operative mortality of 5.4% in this setting.18
Operative approaches
Principles of paraoesophageal hernia repair
The repair of paraoesophageal hernias may be approached via thoracotomy, laparotomy or laparoscopy. The principles of proper surgical repair are the same with each approach:19–21
Laparoscopic repair
Since its introduction by Cuschieri et al. in 1992,22 laparoscopic paraoesophageal hernia repair has gained popularity and proven to be feasible, safe and effective.23–30 Laparoscopy provides an attractive option because it combines some of the advantages of both thoracotomy (access to the hiatus and ability to perform extensive mobilisation of the oesophagus under direct vision) and laparotomy (lower morbidity and no need for single-lung ventilation or postoperative chest tube). Further, this minimally invasive approach may be better suited for the elderly patients in whom this disease most commonly occurs. This is a technically challenging operation that requires advanced laparoscopic skills, but when performed properly, carries a recurrence rate similar to the open approach.31
Set-up and port placement
A total of five trocars are used (Fig. 17.2). Peritoneal access is gained using a Veress needle, and an 11-mm trocar is placed just left of the midline, 15 cm caudal to the xyphoid process. Two ports are placed in the left subcostal position, a 12-mm trocar 12 cm lateral to the xyphoid process, and a 5-mm trocar 8 cm further to the left. Finally, two 5-mm ports are placed along the right costal margin, one in the subxyphoid position and the second approximately 10 cm lateral. A Nathenson liver retractor is used to retract the left lobe of the liver and expose the oesophageal hiatus.
Reduction of hernia sac and fundic mobilisation
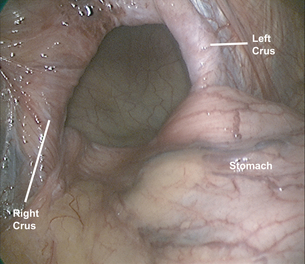
The stomach is gently reduced from the hernia sac (Fig. 17.3) and the left diaphragmatic pillar is identified. The dissection is carried out along the left crus between the endothoracic fascia and the hernia sac. After the anterior phreno-oesophageal membrane is divided using ultrasonic dissection, the stomach is retracted to the left to expose the right crus. The pars flaccida of the lesser omentum is divided and the dissection continues along the right crus to the decussation of the crura. The gastrosplenic omentum is then divided using ultrasonic dissection, and the short gastric vessels are divided all the way up to the cephalad extent of the stomach. Division of the posterior gastric attachments creates a retro- oesophageal window through which a Penrose drain is passed around the oesophagus to allow caudal retraction on the oesophagus and create exposure for the mediastinal dissection. Dissection proceeds from right to left, and the sac is opened to reveal a plane between the peritoneal sac and the mediastinum. Careful blunt and ultrasonic dissection develops this plane, with care taken to identify and preserve the vagal trunks and the peritoneal coverage of the crura, which aids in a second crural closure. With gentle retraction, the sac can be slowly mobilised out of the mediastinum and reduced into the abdominal cavity. Most surgeons excise and remove the hernia sac, but excessive fastidiousness in this exercise risks injury to the vagi as they course in close apposition to the hernia sac in the epiphrenic fat. We usually remove the majority of the sac, including all sac and epiphrenic fat on the left side of the oesophagus, well away from the vagal trunks and the end branches of the left gastric artery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree