Fig. 5.1
Daily volumes of fluid entering and being absorbed by the gastrointestinal system, and excreted into feces. Fully 98 % of the fluid load is absorbed in the intestine, with only 200 g/day or less excreted via the stool. Compromise of absorption efficiency results in diarrhea (Adapted from American Gastroenterological Association Teaching Slide Collection 25 ©, Bethesda, Maryland; Used with permission)
2 Organization of the Intestinal Mucosa
2.1 Intestinal Cells and Factors Involved in Water and Electrolyte Transport
The transport of water and electrolytes by the intestinal mucosa involves several types of cells and structural relationships. Important components of this process are discussed below.
Epithelial Cells
Epithelial cells represent the largest population of cells of the intestinal mucosa , of which there are four major types: (1) columnar, polarized epithelial cells, capable of vectorial transport of nutrients and electrolytes; (2) mucosal endocrine cells; (3) mucus producing and secreting goblet cells ; and (4) defense-producing Paneth cells located at the base of intestinal crypts (Fig. 5.2). The latter two cell types will not be discussed, as their role in intestinal water and electrolyte transport is questionable or unknown. Gut epithelial cells emanate from a stable stem-cell population located near the base of the crypt and, with the exception of Paneth cells, differentiating as they migrate up the crypt-villus axis. As the cells reach the villus tip, they undergo apoptosis (physiologically programmed cell death ) and are sloughed off into the lumen, with the entire sojourn taking 3–5 days. As cells undergo crypt-to-villus differentiation, significant changes in cellular morphology are evident (Fig. 5.3a), characterized by increasing polarity of the cells, differences in cellular organelle components, development of the microvillus membrane and terminal web, and alterations in other cytoskeletal and tight-junctional structural features. The latter are accompanied by decreases in junctional permeability, a property that may arise out of necessity to achieve efficient absorption of nutrients and electrolytes with a minimum of back flux. The regional differences in tight-junction permeability are reflected by the number of intercellular strands that make up the tight junctional complex. The number of strands in this anastomosing network in villus cells is much greater than in the crypt cells , making the villus regions more impervious to passive diffusion of water and electrolytes.
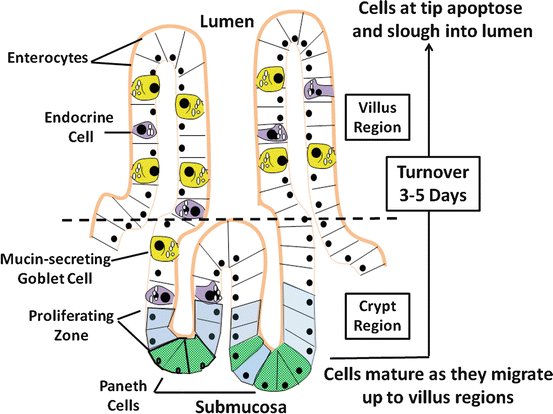
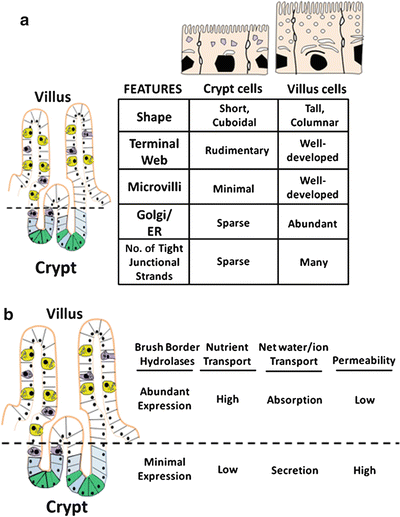

Fig. 5.2
Absorptive and secretory flows that determine the next fluid movement of the intestinal epithelial cells . There are four major types of epithelial cells making up the intestinal mucosa: enterocytes, endocrine cells, goblet cells , and Paneths cell. With the exception of Paneths cells, cells originating from the proliferative zone migrate up the villus axis and mature during the process, which eventually have a turnover rate of 3–5 days

Fig. 5.3
Changes in structures and functions of villus cells and crypt cells. (a) As cells undergo crypt-to-villus differentiation, significant changes in cellular morphology become evident. (b) Alterations of functional characteristics of enterocytes occur along the crypts-villus axis (Adapted from American Gastroenterological Association Teaching Slide Collection 25 ©, Bethesda, Maryland; Used with permission)
Alterations in functional characteristics along the crypt-villus axis also occur (Fig. 5.3b). Absorption and secretion of water and electrolytes are two distinct processes in the gut that take place in the villus and crypt cells , respectively. Cystic fibrosis transmembrane regulator (CFTR), a cyclic adenosine monophosphate (cAMP) regulated Cl− channel involved in anion secretion, is predominantly found in crypt cells, consistent with a secretory role. As these cells become villus cells , the protein synthetic and secretory capabilities increase, reflected by increased development of the Golgi-endoplasmic reticulum complex and numbers of secondary endosomal vesicles. Properties of nutrient absorption and digestion appear, exemplified by increases in brush-border hydrolase activities, glucose transport and increased surface area of the apical membrane. Thus, the observed morphological, phenotypical and functional changes assumed by differentiating villus cells are consistent with their enhanced capacity for absorption of nutrients and electrolytes.
Blood Capillaries and Lymphatics
Blood capillaries and lymphatics have a major role in intestinal water and electrolyte transport. During absorption, for instance, they rapidly remove absorbed nutrients, water and electrolytes from the interstitium, thereby allowing vectorial transport to proceed. Similarly, active secretion of water and electrolytes is accompanied by increased mucosal blood flow and capillary filtration and by decreased villus lymph pressure and total lymphatic flow. In each case, in order for efficient absorption and secretion to occur, the events of mucosal transport, capillary flow, and lymphatic function must be coordinated. Neural and hormonal agents play a major role in the integration of these events. During secretion, for example, the net effect of these agents is to increase delivery of plasma fluid and electrolytes to match the demands of active intestinal secretion. Another purpose of increased blood flow during active secretion is to increase tissue delivery of oxygen to meet metabolic demands.
Intestinal Motility
The relationship between intestinal motility and mucosal water and electrolyte transport is extremely complex and incompletely understood. Although the functional properties of motility and ion transport can be readily studied independently, their physiological roles in intestinal water nutrient and electrolyte transport in vivo are very much interdependent. This was recognized as early as 1912 by Babkin and Ishikawi, who noted that the periodicity of intestinal secretion coincided with that of intestinal motor activity. Consistent with this notion, there is ample evidence that enteric reflexes coordinate intestinal water and electrolyte secretion with smooth muscle contractions (Fig. 5.4). The integration of these responses serves several important purposes. First, it provides an immediate response to begin the process of digestion and absorption of luminal contents. Increased net secretion is essential for providing the aqueous milieu to reach isotonicity and for digestive enzymes to function properly. Coordinated patterns of intestinal motility ensure that mixing and propulsive activity of luminal contents proceed appropriately. This mechanical activity can significantly enhance the absorption of nutrients, electrolytes, and water through several potential mechanisms. Segmentation of the gut helps mix luminal contents with secretions and digestive juices and increases contact time between the luminal phase and the absorptive mucosal surface. Increased intestinal motor contractility is believed to alter the unstirred water layer, present as the result of the overlying layer of mucus gel. This facilitates diffusion of nutrients and electrolytes to the transporters of the brush-border membranes.
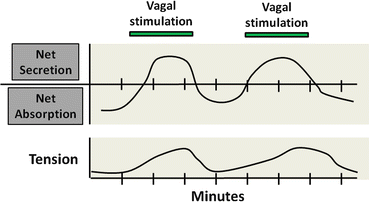

Fig. 5.4
Neural integration of intestinal motility and secretion. Coordination of water and electrolyte transport with intestinal motor function. Enteric reflexes coordinate intestinal water and electrolyte secretion with smooth muscle contractions
Decreased propulsive motor activity may enhance contact time between the absorptive surface area and luminal contents and is probably the major mechanism of action of commonly used antidiarrheal agents. In clinical studies of loperamide, codeine, and the α2-receptor agonist clonidine, the predominant proabsorptive effect of these agents appeared to be due to their ability to increase the “enteropooling” capacity of the gut. The net effect of this action is to increase contact time between luminal fluid and the absorptive surface area.
2.2 Mucosal Cells Involved in the Regulation of Gut Water and Electrolyte Transport
Mucosal Endocrine Cells
Endocrine cells are widely distributed in the intestinal mucosa and represent a major source of active amines and polypeptide hormones important in the regulation of fluid and electrolyte transport. Although many of the contents of their secretory granules are also found in nerve cells of the enteric nervous system , it is likely these cells have unique and independent role in modulating various mucosal functions. Like their transporting epithelial counterparts, mucosal endocrine cells are polarized. Their apical or luminal pole is characterized by tufts of microvilli and coated vesicles, whereas their secretory granules and nuclei are located at the basal domain of the cell. These structural characteristics most likely represent the functional requirements of the cell to sense alterations in luminal content such as pH, osmolality, and chemical content and initiate an integrated mucosal response through the release of secretory-granule contents at the basal surface of the cells. Several peptides, active amines, and other agents appear to modulate intestinal fluid and electrolyte transport (Fig. 5.5). The regional distribution of these agents throughout the gastrointestinal (GI) tract differs markedly. For example, cells containing gastrin , cholecystokinin (CCK), and secretin are more prominently found in the stomach and proximal small intestine , whereas neurotensin -containing cells are largely restricted to the ileum . Serotonin-containing enterochromaffin cells are found throughout the mucosa but predominantly in the crypt regions, where they may project basal processes that run subjacent to neighboring epithelial cells and nerves containing other peptides. Recently, guanylin, the natural endogenous peptide agonist of the E. coli heat-stable enterotoxin (STa) receptor, has been localized to epithelial (nonclassic endocrine) cells of the colonic mucosa (and possibly Paneth cells of the human small intestine). Guanylin is released into the crypt lumen and stimulates luminal receptors on intestinal epithelial cells by a paracrine action to stimulate net secretion.
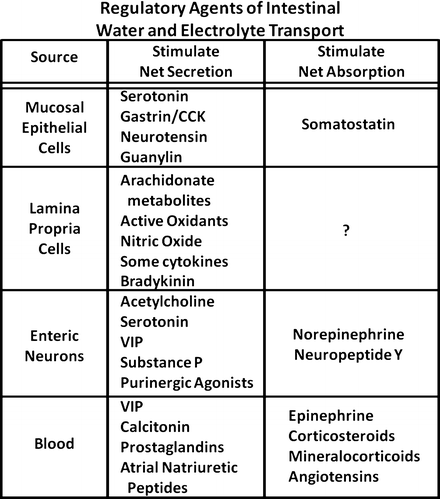

Fig. 5.5
Common regulators for the control of gut water and electrolyte transport. There are peptides, active amines, and other agents from different gut layers that can modulate intestinal fluid and electrolyte transport
Mucosal endocrine cells most likely regulate intestinal ion-transport functions through a paracrine action. They can be activated by a variety of luminal and serosal stimuli. Thus, mucosal endocrine cells probably serve two important functions relevant to the control of intestinal water electrolyte transport. First, they provide a means for the mucosa to sense and rapidly respond to alterations in luminal content and milieu. The stimulated release of hormonal peptides and active amines causes appropriate changes in ion-transport functions, blood and lymphatic flow, and intestinal motility. Secondly, they may function as a fine tuning mechanism or amplifier for modulatory signals received from neural sources.
Enteric Neurons
The GI tract is one of the most richly innervated organs of the body and has two major categories of nerves: the intrinsic or enteric nervous system and the extrinsic autonomic nervous system consisting of parasympathetic and sympathetic nerve pathways. Although most postganglionic sympathetic fibers terminate in enteric ganglia, a few have been reported in close proximity to intestinal epithelial cells, where they may form actual synapses. Release of norepinephrine from these neurons stimulates α2-adrenergic receptors on the basolateral membranes of enterocytes, causing increased electroneutral absorption of sodium chloride and inhibition of anion secretion. Parasympathetic neurons are also believed to be important in the regulation of intestinal salt and water transport. However, the nature of vagal postganglionic fibers to the intestine is less well understood. Some fibers make up interneurons that modulate enteric system tone or responses.
The enteric nervous system is a dense plexus of efferent, afferent, and interneurons , exceeding in number the neurons of the spinal cord. It is composed of cholinergic and non-cholinergic neurons that regulate numerous mucosal and motor functions. The number of neurotransmitters found in these nerves is quite large and includes active amines (such as serotonin and acetylcholine ), neuropeptides (such as substance P, neurotensin , CCK, neuropeptide Y , somatostatin , calcitonin gene-related peptide , vasoactive intestinal peptide and galanin ), and purinergic neurotransmitters (such as adenosine and adenosine triphosphate, ATP). This tremendous diversity of neurotransmitters is probably required to regulate and coordinate the numerous mucosal and motor functions involved in salt and water transport. Many of the enteric nervous system neurons are part of programmed reflexive circuits that can immediately respond to various stimuli. Mucosal sensory fibers respond to a number of stimuli, including mechanical factor (touch, pressure, and tension), changes in luminal content and composition (pH, osmolality, and amino acids), temperature, and pain. These sensory signals are then relayed to interneurons that rapidly process and sort the signals so that efferent (motor) neurons (regulating smooth muscle, blood vessel, absorptive and secretory cells, and other cells of the lamina propria ) can produce patterned and coordinated responses for efficient transport of water and electrolytes. The enteric nervous system plays a major role in regulating intestinal water and electrolyte transport, as evidenced by the fact that mucosal and motor functions can proceed independently of extrinsic neural input.
Mesenchymal Cells of the Lamina Propria
Mesenchymal cells of the lamina propria and submucosa play a juxtacrine role and modulate intestinal mucosal transport, blood flow, and motor functions. They include many cell types such as sub-epithelial fibroblasts , endothelial cells, mast cells , neutrophils , macrophages, and eosinophils. Their numbers within the mucosa vary considerably depending on the species, the region of the intestine, and the prevailing physiological or pathophysiological circumstances. Although their relative roles in the physiological regulation of intestinal water and electrolyte transport have not been established, it is likely that many of these cells play a major role in causing net secretion, altered motor function, and changes in blood flow under pathophysiological situations such as mucosal inflammation.
Many of these cells appear to have important interactions with neural and epithelial elements within the intestinal mucosa (Fig. 5.6). For example, mast cells , found throughout the intestinal mucosa and often in close proximity or juxtaposed to enteric nerve fibers, activate enteric neurons. This serves to amplify and extend the effects of mast-cell mediators such as histamine . These cells have an important immunoregulatory role and are involved in the allergic and anaphylactic reactions to food antigens and helminth parasites, as well as in diseases such as systemic mastocytosis . The number of inflammatory mediators released by the mast cell is large and includes agent such as histamine, adenosine, platelet-activating factor (PAF), serotonin, and arachidonic acid metabolites. Agents such as prostaglandin E 2 (PGE 2 ), adenosine, and serotonin stimulate net intestinal secretion in part by directly activating epithelial receptors, causing decreased absorption of Na+ and Cl− and activate secretion of anions. However, these agents and others such as histamine and PGD 2 also stimulate net secretion by promoting the release of various neurotransmitters, thus amplifying or augmenting the overall secretory response.
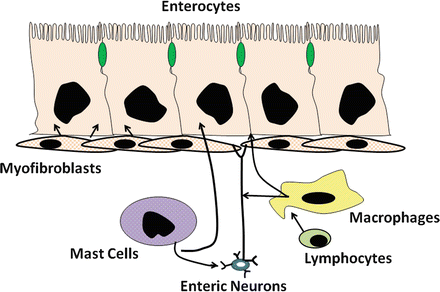

Fig. 5.6
Interaction of enteric cells and other mucoal cells for intestinal water and electrolyte transport. Mesenchymal cells of the lamina propria play an important role in regulating intestinal water and electrolyte transport (Adapted from American Gastroenterological Association Teaching Slide Collection 25 ©, Bethesda, Maryland; Used with permission)
Resident tissue macrophages are also prevalent in the intestinal mucosa, constituting 10–20 % of the total cell number in the lamina propria . This makes the small and large intestine one of the largest repositories of macrophages in the body. Under steady-state and physiological conditions, there appears to be a constant turnover of macrophages (on the order of days to weeks), mostly from the replacement of existing tissue macrophages with incoming monocytes. In the intestine, the macrophages may be conditioned by tissue-specific influences, such as bacterial products from the lumen (including endotoxin), extracellular matrix, and cytokines. The continuous exposure to foreign antigens and pathogens, particularly in the colon, may be important in sensitizing macrophages, allowing them to rapidly react to various stimuli. Thus, macrophages function as a first-line defense against pathogens and antigens and are capable of orchestrating and amplifying an appropriate immune and inflammatory response. The intestinal macrophages are veritable factories for synthesis and release of numerous immune and inflammatory mediators. They are a major source of carbon monoxide and 5-lipoxygenase (5-LO) metabolites and may mediate the secretory effects of many of the secretagogues that are known to activate the arachidonic acid cascade . In response to numerous immune and inflammatory stimuli, they also elaborate cytokines such as interleukin-1 (IL-1), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and other inflammatory mediators such as purinergic agents. Most of these products have now been shown to affect intestinal water and electrolyte transport and have potent effects on modifying intestinal motor functions and capillary blood flow and permeability. Like mast cell products, they have numerous sites and mechanisms of actions that affect ion transport. These will be discussed later in the chapter.
3 Mucosal Electrolyte Transport Processes
3.1 Absorptive Pathways for Water and Electrolytes
Water transport by the intestine is closely coupled with solute movement and is passive. In theory, water flow could occur by transcellular and paracellular routes, but the prevailing evidence indicates that water transport by the intestine occurs through the paracellular pathway . Like other tissues that transport electrolytes, the intestine has a variety of specialized transport proteins, which can be divided into three major types (Fig. 5.7).
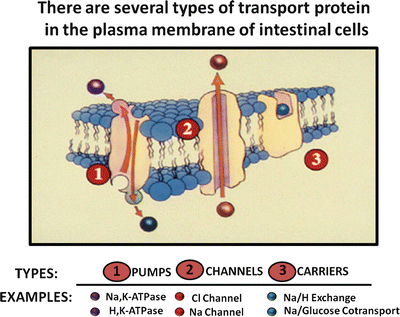

Fig. 5.7
Intestinal transport proteins. There are three types of transport proteins that are found in the plasma membranes of intestinal cells: pumps, channels and carriers (Adapted from American Gastroenterological Association Teaching Slide Collection 25 ©, Bethesda, Maryland; Used with permission)
Pumps such as sodium pump (Na+/K+-ATPase) and the proton pump (H+/K+-ATPase ) are energy-driven and capable of transporting ions against large electrochemical gradients. In intestinal epithelium, for example, the sodium pump is essential for establishing and maintaining electrochemical gradients (low intracellular Na+ and electronegative membrane potential) that are required for other types of passive and facilitated transport processes.
Channel proteins are selective membrane “pores” for ions such as Na+ and Cl−. Channel transport is dependent on favorable electrochemical gradients, is often membrane-potential sensitive, and is generally electrogenic, i.e. causing a potential difference across the epithelial layer that promotes passive diffusion of a counter ion. For example, electrogenic Cl− secretion in the gut causes a potential difference (serosa is positive relative to lumen) across the mucosa that promotes passive transport of Na+, resulting in net sodium chloride secretion.
Carrier transport proteins facilitate transport of ions and nutrients across the cell membrane, and their transport activity is dependent on existing electrochemical gradients. Several types of carrier proteins exist in gut epithelium. Uniport carrier proteins, such as the facilitated glucose transporter GLUT2, mediate the transport of a single ion or nutrient molecule; symport carrier proteins, such as sodium/glucose cotransport-1 (SGLT-1 ) protein, are carriers that simultaneously transport two or more molecules, often taking advantage of favorable electrochemical gradients for one molecule to actively transport others. Transport by these carriers occurs only if all solutes are present. In addition, glucose transport will not occur if an inwardly directed Na+ gradient is absent; antiport carriers, such as the Cl − /HCO 3 − and Na + /H + exchangers, exchange one molecule for another.
Absorptive Pathways for Electrolytes
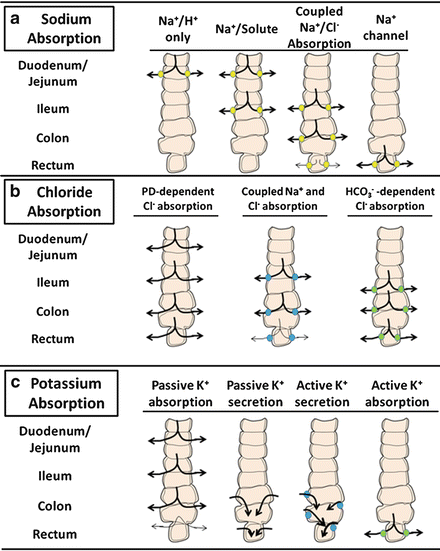
Na + and Cl − are avidly absorbed by the intestinal mucosa , albeit differing in amount and by region-specific transport mechanisms of the gut. Several of these pathways and their relative distribution along the horizontal axis of the intestine are illustrated in Fig. 5.8.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








