Fig. 28.1
(a) Fistula with seton in place (Courtesy of W. Brian Sweeney, MD). (b) Fistulotomy with marsupialization of the tract (Courtesy of W. Brian Sweeney, MD)
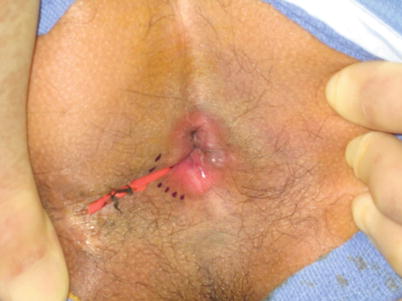
Fig. 28.2
Seton in place in a fistula in ano
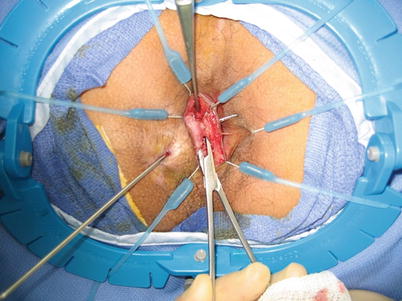
Fig. 28.3
Fistula tract dissected out in the intersphincteric space
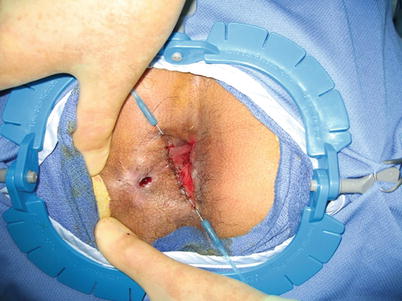
Fig. 28.4
Closure of the fistula tract
Transanal endoscopic microsurgery (TEM) has been accepted as an alternative to surgery for the treatment of early rectal cancers with improved functional results being an important motivating factor (see Video 14.1). Patients undergoing TEM were assessed over 60 months of follow-up and found to have early worsening of continence, urgency, and quality of life with a return to baseline or better over the course of the follow-up [27]. Overall, risks of altered bowel function after anorectal surgery must be individualized to the patient, procedure, surgeon, and underlying disease.
Prolapse Surgery
Key Concept: Functional outcomes following correction of rectal prolapse depend on both the procedure performed (rectopexy, resection–rectopexy, perineal procedures) and the preoperative condition of the patient (i.e., constipation vs. incontinence).
More than 100 different operations have been described for prolapse; therefore, it is not surprising to see rates of functional problems following prolapse repair to be widely variable. While several different mechanisms contribute to normal defecation, abdominal prolapse procedures risk damage to nerves controlling defecation, while perineal procedures remove the rectum with resultant loss of its intrinsic capacitance and reservoir. Additionally, some patients preoperatively suffer severe alterations to anorectal physiology that may prove recalcitrant to surgical improvement. A prospective analysis of 86 patients over 36 months of follow-up after ventral rectopexy demonstrated improvement in continence in 68 % of patients. However, the authors noted that 50 % of patients remained incontinent postoperatively. Older patients with longer duration of prolapse and worse preoperative continence scores were more likely to be incontinent postoperatively [28]. Perineal operations remain a viable option for debilitated patients with rectal prolapse, but data on functional outcome are variable. In one study, 6-month follow-up after Altemeier procedure, i.e., perineal rectosigmoidectomy, revealed that 62 % of patients demonstrated improvement in continence, although 25 % remained incontinent [29]. In contrast, only 28 % of patients had improved continence after Altemeier procedure in long-term follow-up of 93 patients, and several had deterioration or de novo incontinence [30]. Prospective trials of perineal versus abdominal approaches to rectal prolapse are forthcoming and may improve the surgeon’s expectations for postoperative functional outcomes.
Management
Key Concept: The approach to functional problems following colorectal surgery should initially be on the exclusion of a potentially reversible technical error, followed by symptom-based therapy directed towards the patient’s individual symptom complex.
We start the treatment of any patient with defecatory dysfunction after surgery by excluding a technical error. Once healed from surgery, the patient should undergo a flexible sigmoidoscopy (for a high anastomosis) or a digital rectal exam (for a low anastomosis) to exclude an anastomotic stricture or proctitis/colitis. If a stricture is found, it should be dilated and the symptoms should then be reassessed post dilatation. In absence of stricture, the treatment can focus on amelioration of symptoms, as described below.
Diarrhea
Key Concept: Most diarrheas can be treated with medical therapy alone through bulking and motility–slowing agents. Excluding/identifying any underlying infection and accounting for any anatomical changes (i.e., short bowel, TI resection) will also aid in the selection of the appropriate medical therapy.
The treatment of diarrhea following colorectal surgery focuses on dietary changes and medications (Table 28.1). These symptoms range in frequency depending on the operation, but the best literature on the topic stems from patients treated with IPAA. Postoperative patients with IPAA make frequent dietary changes to control stool frequency and consistency. In a survey of 64 postoperative patients, the vast majority identified specific food triggers of increased stool frequency, decreased consistency, and perineal irritation; 61 of 64 surveyed obeyed a strict dietary regimen [31]. Dietary restrictions were also found in patients following pelvic radiotherapy, with elimination of raw vegetables seeming to be the most helpful measure [32]. Long-term dietary restrictions, in combination with bacterial overgrowth and functional alteration of the terminal ileum, can yield important nutritional deficiencies, specifically vitamin B12, iron, vitamin E, and fat malabsorption [33]. The presence of anemia or other signs and symptoms of malnutrition should prompt investigation and oral supplementation.
Table 28.1
OTC agents for symptom management
Name | Dose | Mechanism |
|---|---|---|
Loperamide | 4–8 mg/day | Opioid agonist—peripheral |
(Imodium) | ||
Diphenoxylate/atropine | 5 mg 4 times/day | Opioid agonist |
(Lomotil) | ||
Tincture of opium | 6 mg 4 times/day | Opioid agonist |
Bismuth | 524 mg as needed, up to 8 doses/24 h | Antisecretory, antimicrobial, antiinflammatory |
(Pepto–Bismol, Kaopectate) | ||
Psyllium | No standard | Soluble fiber, bulking agent |
(Metamucil, Konsyl, Reguloid) |
The staple of medical treatment for diarrhea following colorectal resections, independent of the size of resection, is therapy designed to reduce gastrointestinal transit time and alter stool consistency. Many patients remain dependent on these agents over the long term. Few of these therapies have any experimental data in any patient population with chronic diarrhea, much less postsurgical patients. Opiates such as loperamide, tincture of opium, and diphenoxylate function to reduce GI propulsion. Loperamide has its effect only in the intestinal muscle, whereas others have the potential for central nervous system activity and are controlled substances. Intraluminal bulking agents such as fiber supplementation and psyllium may provide improved stool consistency to some patients. Bismuth has some utility in nonspecific chronic diarrhea and may provide relief to some patients.
Development of new drugs has been limited. Serotonin receptor antagonists were found to be associated with ischemic colitis and calmodulin therapies demonstrated no superiority to loperamide [34]. Octreotide, which has been found of utility in other forms of chronic diarrhea, was tested in a small randomized, placebo-controlled trial. It demonstrated no improvement in bowel frequency in patients with post-IPAA diarrhea and a potential increase in painful tenesmus, causing two patients to withdraw from the study [35].
One potential new therapy is probiotic bacterial cultures. Probiotics are postulated to improve gastrointestinal symptoms by modifying the immunologic, digestive, or nutritional functions of commensal gut bacteria. Treatment with probiotics in multiple formulations has been studied in a variety of gastrointestinal conditions. Utility has been demonstrated in infectious diarrhea and antibiotic-associated diarrhea, but study data have been less convincing in IBD and irritable bowel syndrome [36]. In the postoperative setting, 67 patients with IPAA due to UC or FAP demonstrated improvement in abdominal cramping, leakage, need for pad use, and involuntary defecation following a 4-week intervention with live Lactobacilli and Bifidobacteria [37]. Mucosal inflammation, scored by endoscopy, was also decreased by the intervention in UC patients. Larger, controlled trials are needed before utility can be shown conclusively.
Ileal resection or disease results in spillover of bile acids into the colon, interfering with electrolyte and water absorption and frequently causing diarrhea. Cholestyramine, colestipol, and colesevelam, bile acid sequestrants, prevent the outpouring of water and electrolytes. In a single-blind prospective trial, cholestyramine reduced stool frequency and volume in patients with ileal resections <100 cm. It demonstrated no improvement in patients with >100 cm resected [38]. IPAA also disrupts the ileum and interferes with enterohepatic circulation, as demonstrated by elevated postprandial serum levels of unconjugated bile acids [39] and abnormal 75Se homotaurocholate uptake in patients following IPAA [40]. Pouchitis, stasis, and bacterial overgrowth may all worsen this condition [41]. Although its role in diarrhea for patients after colectomy or IPAA is not well established, cholestyramine may provide relief to some patients suffering from diarrhea and has demonstrated efficacy in alleviated perianal skin irritation following IPAA [42]. Additionally, any suggestion of pouchitis (i.e., abrupt increase in watery stools, fever, pelvic pain) should prompt an endoscopic evaluation of the pouch, biopsy, and likely empiric treatment with antibiotics such as Flagyl and/or Floxin.
To summarize, our algorithm for the treatment of diarrhea is to always perform a colonoscopy or a flexible sigmoidoscopy first to evaluate the colon and exclude ischemic or inflammatory colitis or an anastomotic stricture. All patients are tested for Clostridium difficile colitis before initiating drug therapy. Whenever possible, patients are asked to stop all antibiotics to make sure the diarrhea is not antibiotic induced. They are then started on a probiotic. All patients after right-sided colectomy or small bowel resections are started on cholestyramine. In the absence of improvement with probiotics and cholestyramine, when appropriate, we then start a fiber supplement, such as Metamucil® (Procter & Gamble) or Benefiber® (Novartis). The patients are asked to start with half the dosage listed on the medicine box and are informed to expect bloating and distention as they adjust to the supplement. In 10 days, the patients are asked to escalate to the dose suggested on the box. Any fiber supplement brand is adequate and we ask the patient to choose the one that he prefers. If fiber fails, we escalate to loperamide. We instruct the patients to take as many as eight loperamide tablets per day to achieve 2–3 formed bowel movements daily. If fiber and loperamide fail, we continue with fiber supplementation and switch to Lomotil (diphenoxylate/atropine). Finally, we reserve prescriptions for diluted tincture of opium (DTO) for desperate cases.
Fecal Incontinence
Key Concept: The treatment of the patient reporting complaints of anal leakage of mucus, gas, liquid, or stool should always start with identification of the underlying cause of their incontinence.
The most common cause of incontinence is not sphincter insufficiency, but diarrhea. Thus, in the patients reporting diarrhea, we always start with its treatment, as described above. In those who continue to have leakage despite adequate regulation of bowel frequency and consistency with bulking agents (fiber) and constipation agents (i.e., Loperamide), we consider a prescription of amitriptyline, which can be added at a dose of 10–25 mg at night as tolerated. Amitriptyline is a tricyclic antidepressant agent that was studied in an open label trial of patients with fecal incontinence and was found to decrease incontinence scores [43]. Seventy-two percent of patients who were treated with the drug in the study reported full remission with a sustained improvement at 6 months.
In patients who continue to do poorly, we proceed with a thorough work-up aimed at excluding fecal obstruction and subsequent overflow incontinence. To start with, we perform a flexible sigmoidoscopy or a colonoscopy to exclude an anastomotic stricture. Once a stricture is excluded, we proceed with anorectal manometry testing to assess for any evidence of a paradoxical contraction of the puborectalis. In the patients who are found to have signs suggestive of this condition, we proceed with treating fecal incontinence with a daily glycerin suppository and a weekly tap water enema. This treatment has been shown to be effective in at least one-third of the patients with this condition [44].
Finally, when all medical therapy fails, we consider surgical therapies that escalate depending on the patient’s interest in proceeding with further treatments and their disease severity, as well as the remaining anatomy and the underlying diagnosis. For example, the patient who has not received pelvic radiation and who does not have Crohn’s disease may be a candidate for receiving a submucosal injection of Solesta® gel (Salix Pharmaceuticals Inc., Raleigh, NC) into their anal sphincter. The gel, which was recently approved by the FDA, has been shown to have a 60 % response rate at a 6-month follow-up, which was nearly twice the improvement rate seen in the placebo group [45].
In the patients who cannot have direct anal sphincter injections, sacral nerve stimulation (SNS) (Medtronic Interstim®, St. Paul, MN) is another great potential option that was also recently approved by FDA in the US. Its only drawback (besides its high price) is the fact that the device is not MRI compatible. The device is only planted, however, after a 2–3 weeks trial of stimulation. Eighty percent of the patients who do well during the stimulation phase can expect a 50 % reduction in the frequency and the severity of their fecal incontinence. Forty percent can expect complete continence [46].
Those who remain incontinent following medical therapy and minimal invasive treatments with either Solesta® or SNS, or both, could consider implantation of an artificial bowel sphincter (ABS), a hidden mini stoma, to perform Malone antegrade colonic enemas (MACE) or a permanent ostomy. I reserve the option of ABS only for the patient who has a colon and has solid bowel movements. Furthermore, the patient’s perineum needs to allow for a safe implantation (i.e., no Crohn’s, radiation, diabetes, immunodeficiency). Similarly, the MACE procedure is only feasible in a patient who has a colon that could then be irrigated to empty. The patients without a colon who fail SNS are unfortunately only candidates for an ileostomy.
Constipation/Obstructed Defecation
Key Concept: Recognize the presence of obstructed defecation in patients with pre– or postoperative anorectal complaints as these will need to be addressed but may prevent unnecessary surgical re–intervention.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








