2
Epidemiology, genetics and screening for oesophageal and gastric cancer
Definitions
The concentration of disease around the oesophagogastric junction has created differences in opinion with regard to classification. This partly reflects differences in the pathological behaviour of tumours arising at the different sites. In addition, the recent change in the TNM classification1 has described cancers as either oesophageal, including all within 5 cm of the oesophagogastric junction, or gastric. In epidemiology it is important to ensure a clear classification in order to understand differences in incidence and to appreciate aetiological evidence for the observed changes in these cancers.
For the purposes of the following discussion, carcinoma of the oesophagus will include cancers of the thoracic and abdominal oesophagus but will exclude the cervical oesophagus. Oesophagogastric junctional cancers will be considered according to the Siewert and Stein classification:2
• Type I is adenocarcinoma of the distal oesophagus, which usually arises from an area of Barrett’s metaplasia and which may infiltrate the oesophagogastric junction from above.
• Type II is true carcinoma of the cardia arising from the cardiac epithelium or short segments with intestinal metaplasia at the oesophagogastric junction, often referred to as ‘junctional carcinoma’.
• Type III is subcardial gastric carcinoma that infiltrates the oesophagogastric junction and distal oesophagus from below.
Non-cardia gastric cancer will include all cancers of the fundus, body and pyloric antrum.
Epidemiology
Oesophageal cancer
Carcinoma of the oesophagus (ICD code 150) was the eighth commonest cancer in 2008.3 Worldwide there were 481 000 new cases representing 7% of the total cases of cancer. Mortality was high, with 406 000 deaths or 84% of all registered cases. Incidence varies across the world, with the highest risk in the so-called Asian ‘oesophageal cancer belt’, which extends from Northern Iran through Central Asia to North Central China. SCC predominates in these less developed countries, reflecting low socio-economic status and poor diet. Overall, the male to female ratio is 2.1:1, although there are variations. In more developed countries the incidence of SCC has declined, with age-specific rates in white males in the USA at 2.2 per 100 000.4 However, there have been increasing trends in some regions; for example, in Scotland rates are increasing in women and decreasing in men, possibly reflecting the changing patterns of tobacco and alcohol consumption.
Oesophageal and oesophagogastric junctional adenocarcinoma
Adenocarcinoma of the oesophagus and junction accounts for variable proportions of oesophageal cancer across the world, ranging from 0% in parts of China to 10% in Northern Europe to 48% in the UK.4 Adenocarcinoma of the oesophagogastric junction includes cancer located in the distal third of the oesophagus and the cardia of the stomach. Although there is a male predominance there are differences according to organ of origin. For oesophageal tumours the male to female ratio is 2.6:1 and for gastric origin tumours 4:1. Recent data from England show an increase in incidence of lower third oesophageal cancer from 8.1 per 100 000 in 1998 to 10.1 per 100 000 in 2007, although the rate of increase has stabilised since 2002.5 Over the same period there has been a slight decrease in cardia cancer incidence. The peak age group affected is between 50 and 60 years of age.
Gastric cancer
Gastric cancer (ICD code 151) is the fourth most frequent cancer worldwide.3 In 2008 there were 988 000 new cases with 737 000 deaths. This represents 14% of all new cases of malignancy and 10.3% of all cancer deaths. Crude numbers are still increasing in relation to demographic changes of the ageing population throughout the world. The majority of cases occur in less developed countries, where the male to female ratio is 1.8:1. This contrasts with a ratio of 1.6:1 in more developed countries. Highest incidence rates are found in Japan (male 69.2 per 100 000 and female 28.6 per 100 000). Other countries with high incidence include East Asia, Korea, Eastern Europe, and Central and South America. Although distal cancers still predominate in countries with highest incidence, there has been a fall in mid and distal gastric cancer, with a progressive increase in cardia cancer.
Aetiology
Squamous cell carcinoma of the oesophagus
Socio-economic and dietary influences
Areas of highest incidence are those countries of low socio-economic status where poverty and malnutrition predominate. The development of SCC appears to be related to a type of chronic oesophagitis that is different from that found in the West and is often complicated by atrophy and dysplasia (see Chapter 1). It is not usually associated with gastro-oesophageal reflux and is often asymptomatic.
SCC has been associated with ingestion of very hot beverages, a family history of oesophageal cancer, prevalence of oesophagitis among siblings, and a low intake of fresh fruits and wheat flour products.6 Furthermore, riboflavin deficiency and vitamin A and C deficiency7 have been identified as risk factors that are particularly important at a young age. By contrast, vitamin C intake confers a protective benefit; Hu et al.,8 in a case–control study, found that 100 mg of vitamin C per day decreased risk by 39%.
Associated conditions
Achalasia is associated with SCC, but the magnitude of the risk is unclear. Brucher et al.9 report from their single institution series that the risk of developing a carcinoma in long-standing achalasia is increased 140-fold when compared with the general population. The risk appears to relate to retention oesophagitis secondary to stasis and exposure to possible carcinogens in fermenting food residue. There is a lead time of approximately 15–20 years and these cases probably warrant long-term surveillance. Treatment of the achalasia does not seem to reduce the risk.
Tylosis palmarum is a rare inherited autosomal dominant condition in which there is a very high incidence of SCC. Perhaps of greater significance is the finding of the increased risk in low-risk areas for offspring of parents with oesophageal cancer.10 There are numerical and structural chromosomal aberrations in patients with a family history not seen in those without a family history (see below).
Adenocarcinoma of the oesophagus and junctional cancers
Gastro-oesophageal reflux disease (GORD)
Gastro-oesophageal reflux is now the most common symptomatic presentation of all conditions affecting the upper gastrointestinal tract. Estimates suggest that 4–9% of all adults experience daily heartburn and up to 20% experience symptoms on a weekly basis.11 Of these, 60% have no endoscopic abnormality, 30% have oesophagitis and 10% have Barrett’s columnar lined oesophagus. Many are self-treated and do not attend for further investigation, yet 80% with Barrett’s are asymptomatic. The relationship of GORD and oesophageal ACA has been evaluated in case–control studies.12 The individual cancer risk is small because of the high frequency of GORD. Lagergen et al.13 have estimated the risk of developing ACA of the oesophagus by scoring symptoms of heartburn and regurgitation (alone or in combination), timing of symptoms (particularly at night) and frequency of symptoms. Among those with recurrent symptoms of reflux, the odds ratio of developing cancer was 7.7 in comparison with those without symptoms. More frequent, more severe and longer-lasting symptoms of reflux were associated with a much greater risk (odds ratio 44). The risk associated with GORD is related to the development of Barrett’s metaplasia, which is greatest among Caucasian males with a history of alcohol consumption and continuous smoking. Further detailed discussion of the role of Barrett’s in the aetiology of ACA is presented in Chapter 15.
Obesity and dietary factors
In the last 20 years the incidence of junctional cancer has increased in parallel with the epidemic of obesity. There is a three- to sixfold excess risk among overweight individuals.14 Obesity predisposes to hiatus hernia and reflux, and hence contributes mechanically to increase risk. However, data from a number of studies demonstrate an effect independent of reflux. Lindblad et al.15 have reported a 67% increase in the risk of oesophageal ACA in patients with a body mass index (BMI) greater than 25, and this increases with increasing BMI. This effect was noted irrespective of the presence of reflux symptoms.
There appears to be a sex difference in that the effect was only found in women with a BMI greater than 30, whereas in men it was observed in both overweight and obese individuals. Recently this effect in women has been confirmed, with 50% of cases of oesophageal adenocarcinoma in postmenopausal women in the Million Women study being attributed to obesity.16
Evidence is accumulating to support different types of obesity. The distribution of abdominal fat tends to be central and retroperitoneal. This acts as a potent source of growth factors, hormones and regulators of the cell cycle. Such individuals develop the metabolic syndrome, which is linked to raised serum cholesterol and triglycerides, hypertension and hyperglycaemia. In the general population the metabolic syndrome occurs in 10–20%. Power et al.17 have demonstrated that 46% of those with Barrett’s oesophagus and 36% of those with GORD have features of the metabolic syndrome.
The factors released by centrally deposited fat may have an effect on the process of metaplasia transforming to dysplasia. Vaughan et al.18 have examined the potential relationship between a series of biological markers of progression from metaplasia to cancer in obese and overweight patients. There was little evidence of change in the biomarkers in association with increasing obesity. However, abnormalities in the biomarkers were observed in individuals with high anthropometric measures of abdominal fat. The study concluded that increased BMI contributed to reflux and development of metaplasia but it was the ‘male pattern’ of abdominal obesity that was actually associated with malignant transformation.
Helicobacter pylori
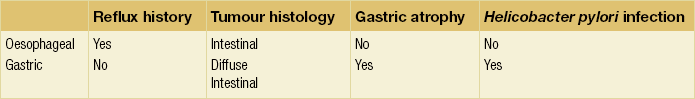
There is accumulating evidence that there may be two distinct types of junctional cancer reflecting the two potential sites of origin. McColl and Going19 have suggested that one is similar to oesophageal cancer and the other gastric cancer. In a series of studies of patients with junctional cancer, they evaluated H. pylori infection from serology, gastric atrophy from pepsinogen I and II ratios, symptoms of reflux and histological subtype of diffuse or intestinal type. They also included biopsies of the distal stomach to document atrophy associated with Helicobacter. Tumours of oesophageal origin are intestinal type with no evidence of gastric atrophy or Helicobacter infection and occur in the context of reflux. By contrast, tumours of gastric origin are diffuse type or intestinal but with evidence of atrophy and Helicobacter infection and without a history of reflux (Table 2.1). Such different characteristics would imply a different carcinogenic process at the two sites and should be considered in prognosis and patient management.
Socio-economic factors
Lifestyle has an effect on the risk for junctional cancers. There is an association with lower socio-economic class but this is not as strong as for SCC. Powell and McConkey20 demonstrated that the increase of ACA of the lower third of the oesophagus and the cardia was mainly in social classes I and II – that is, in professional and managerial occupations. In addition, in a large surgical series, Siewert and Ott21 reported that patients with ACA were more frequently from an educated background, a characteristic not present in the population with SCC. However, the effect of socio-economic class may not be independent as, when adjusted for GORD, BMI and smoking, Jansson et al.22 found the effect to be less apparent.
Gastric cancer
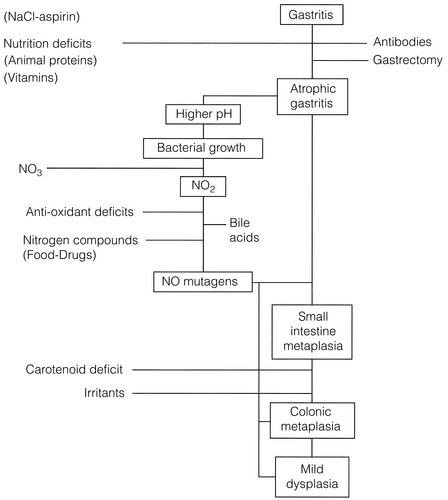
The Correa hypothesis23 (Fig. 2.1) describes the steps in the process of malignant transformation for gastric cancer. It highlights where environmental factors stimulate changes, particularly in the development of intestinal-type gastric cancer (see Chapter 1). These include socio-economic and dietary influences, as well as exposure to carcinogens.
Socio-economic influences
Exposure to potentially carcinogenic agents at an early age is clearly crucial to the risk of developing both precursor lesions and subsequent gastric cancer. Evidence for this risk is available from migrant studies. Japanese migrants to the USA had a far lower rate of intestinal-type cancers than the equivalent population who remained in Japan, indicating environmental or dietary aetiology, whereas the rates of diffuse-type cancer remained the same, suggesting a hereditary component.24
Diet
Salt preservation of food was common during the early years of the 20th century throughout the world; in some landlocked parts of the world this still occurs. In such areas and in those still using salt preservation there have been high rates of gastric cancer. The consumption of salted and pickled fish is high in Japanese and Colombians and correlates with their disease incidence. On the basis that salt induces injury to the gastric mucosa it may act like high carbohydrate intake, as an initiator to allow access for more potent carcinogens. By contrast, the rapid and widespread adoption of refrigerators in the 1950s and 1960s has significantly affected the preservation of fresh foods. The reduction in mortality observed in Japan shows an inverse relationship with the increase in ownership of domestic refrigerators.25
Helicobacter pylori
In 1994 the International Agency for Research on Cancer designated H. pylori to be a type I carcinogen26 for gastric cancer. The initial effect of H. pylori is acute inflammation. Since the infection does not resolve spontaneously, an effect is likely to persist and may proceed to chronic gastritis and associated mucosal atrophy and intestinal metaplasia, dysplasia and eventually cancer. The evidence for its role is from a number of sources. Areas of high cancer incidence have a high rate of H. pylori infection. In a prospective population-based study in Japan, 2.9% of those infected developed gastric cancer compared with none from the uninfected population; 4.7% of those infected who had non-ulcer dyspepsia progressed to cancer.27 In high incidence areas H. pylori infection tends to occur early in life. These early rates of infection are linked to low income, poor education, poor sanitation and overcrowding. There has, however, been a progressive fall in rates of H. pylori serology positivity in longitudinal studies, which have paralled the decline in gastric cancer incidence.
Although the evidence for H. pylori inducing gastric cancer is convincing, not all those infected develop the disease. The risk of malignant transformation appears to be enhanced by bacterial virulence and host factors (see below). Helicobacter pylori with cytotoxin-associated gene A (cagA) appears to be associated with the greatest risk.28 In the West, 60% of H. pylori infections are cagA positive compared with 100% in Japan.29,30 It is likely that H. pylori induces an environment that is susceptible to malignant transformation. It induces tissue monocytes to produce reactive oxygen intermediates, which are potent carcinogens. Infection is associated with a significant reduction in gastric juice ascorbic acid,31 which acts to scavenge and suppress N-nitroso compounds and oxygen free radicals. It also facilitates the proliferation of nitrosating bacteria, which promote the development of N-nitroso compounds.
Prevention of oesophageal and gastric cancer
In oesophageal and gastric cancer primary prevention approaches are currently limited to population education to alter social habits (such as decreasing or stopping tobacco or alcohol consumption) and dietary habits (such as maintaining a diet containing fresh fruit and vegetables with a low or minimal salt intake). In addition, the need to prevent obesity is now well established. The role of H. pylori eradication is important but programmes of eradication should only be considered according to the level of risk for oesophageal or gastric cancer in the population. In populations with a high risk of gastric cancer, eradication is indicated; however, in populations in which oesophageal ACA is common, eradication may have an adverse effect. The overall benefit of these approaches would be greatly enhanced if specific markers of risk could be identified to focus prevention strategies (see Chapter 15).
Secondary prevention depends upon understanding the natural history and detection of premalignant conditions. In SCC there is limited evidence that secondary measures could be effective because of lack of understanding of the histological changes leading to cancer. In oesophageal ACA, surveillance of Barrett’s metaplasia to identify progression to dysplasia is theoretically a positive approach (see Chapter 15). Identification of p53 expression and aneuploidy in biopsies of Barrett’s has been shown to predict the risk of progression.32 In both gastric and oesophageal cancer there is a potential role for chemoprevention. Increasing levels of cyclo-oxygenase-2 (COX-2) are present in the progression of atrophic gastritis to intestinal metaplasia and gastric cancer.33 Smoking, acid and H. pylori are all associated with COX-2 expression. Aspirin and other non-steroidal agents inhibit COX-2 and their use may act as a chemopreventive for gastric cancer. Aspirin also seems to have an effect in Barrett’s metaplasia and in combination with acid suppression may minimise progression to dysplasia. The ASPECT trial in the UK is assessing whether such a strategy can have a secondary preventive effect.34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree