Fig. 7.1
Mortality of secondary peritonitis patients, divided based on the severity of disease expressed by the APACHE-II score. Two surgical strategies were compared per category, re-laparotomy on-demand (□) and planned re-laparotomy (■). A total of 510 patients were registered; patients with an APACHE-II score >10 were randomized in two strata (11–20 and >20) between on-demand and planned re-laparotomy strategy
The acute phase of abdominal sepsis may at some point lead to the development of an enterocutaneous fistula or even several fistulas. Factors that contribute to this unfortunate course of disease are:
Ongoing peritonitis, in particular when combined with multiple laparotomies within a short period of time
A bowel anastomosis in situ
Open abdomen
Inadequate drainage of intra-abdominal abscesses or infected fluid collections
Synthetic meshes in contaminated environment used as bridging or inlay and meshes of whatever material when positioned as an inlay
When an enterocutaneous fistula has developed, it is important to verify whether there is an ongoing intra-abdominal infection. Certainly in the case of a fistula that has a tract from intestine to abdominal wall, remaining abscesses of infected fluid collections have devastating effects. Imaging work-up in this situation should be performed by contrast-enhanced computed tomography (CT). Intravenous contrast is needed in a septic patient, even when renal function is compromised, since an inadequate diagnosis is in the end more harmful than the risk of increased renal insufficiency related to use of contrast. Use of oral contrast that can be applied via the nasogastric tube can enhance CT accuracy in specific cases, although the gain in diagnostic accuracy is limited. Therefore, paralytic ileus that frequently accompanies abdominal sepsis—hampering enteral contrast work-up—should not delay CT imaging.
Thus far, reliable CT accuracy data come from patients suspected of having secondary peritonitis after elective abdominal surgery. The positive predictive value of CT to detect an abdominal source of sepsis (ruling in) is 71 % (95 % CI 57–83 %), leaving a margin of error. However, the negative predictive value for an abdominal source of sepsis (ruling out) is 15 % (95 % CI 6–32 %) making it a reliable modality [14]. In contrast, there are no data on the accuracy of CT after an initial operation for peritonitis and none in the setting of an open abdomen. For fistulas in an open abdomen setting, without any tract, imaging of the fistula is a less pressing matter initially. Harm has been done with early aggressive attempts at closing the fistula, and spontaneous closure of open abdomen fistulas is anecdotal. It is more important to focus on sepsis control.
Overall, antibiotics are usually not indicated merely because of fistulas. Use of antibiotics after the initial event of abdominal sepsis should be reserved for ongoing or new-onset peritonitis or during (percutaneous) drainage in a septic patient, preferably based on previous culture results and resistance pattern.
Managing Patient Expectations and the Importance of “Patience”
Key Concept: Surgeons need to avoid the temptation to rush patients back to the operating room for an attempt to close the enterocutaneous fistula. Explaining the usual extended timeline to the patient and family will be helpful to controlling emotions and managing expectations.
Fistulas usually develop after multiple laparotomies, in an open abdomen, and days after the initial septic event. As a rule of thumb, re-laparotomy for a fistula more than 7–10 days and less than 6 months after previous laparotomy, after multiple recent re-laparotomies, or in case of an open abdomen, is not advised. This means that, in general, the moment intestinal fluid is seen coming out of the laparotomy wound (after fascial closure), a drain, an old drain opening, or the open abdomen, surgery is not an option at that time. This should be made clear to the patient, family, and other involved doctors such as intensivists. Although tempting, any additional surgery in the first phase of a fistula to treat this fistula is a futile attempt and should be avoided. Remember that “early fistula surgery is for the surgeon not for the patient; we surgeons need to be patient.”
Initial fistula management comprises restoring and monitoring of fluid and electrolyte balance, adequate nutrition, and wound care. Apart from CT in case of clinical suspicion of ongoing infection, no imaging of the fistula tract is needed or desirable. The only exception to this rule is leakage or fistulas from the proximal gastrointestinal tract, such as from a surgically closed duodenal perforation or biliary leakage such as leakage from a hepaticojejunostomy or after cholecystectomy. In these cases a percutaneous transhepatic catheter (PTC) drainage is very effective, and after cholecystectomy endoscopic retrograde cholangiography (ERC) and stenting may provide source control.
Wound management should focus on skin protection, using modern wound management systems, which collect fistula fluid and at the same time allow granulation and (near) closure of the wound. Figure 7.2 shows an example of inadequate wound management in the presence of a fistula. Other ways to protect the skin are reduction of fistula production and fluid composition (see section “Rehabilitation phase”).
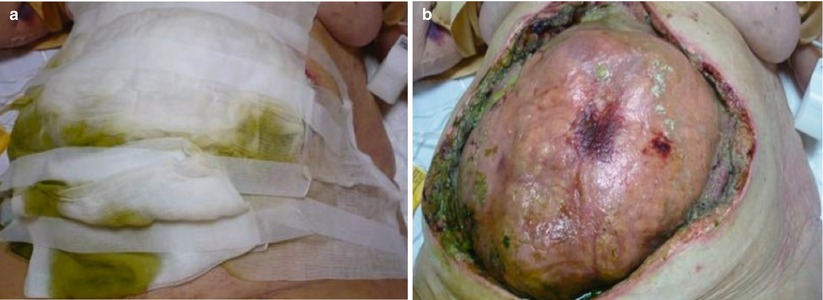

Fig. 7.2
Inadequate wound management of fistula in open abdomen
Evaluation of the Fistula
Defining the Anatomy
Key Concept: In the phase II stabilization period, in absence of ongoing sepsis, defining the fistula anatomy can be performed with the aid of a small bowel contrast studies, CT, or preferably, MR enteroclysis.
During phase I (≤2 days after its presentation) of enterocutaneous fistula management, focus is on its diagnosis and evaluation of coexisting ongoing peritonitis, as has been described in the previous paragraph. The diagnosis of a fistula can be relatively simple if evident bowel content is seen in wound or drain or open abdomen. Usually we can deduce the most likely cause of this leakage, i.e., anastomotic leakage, leakage from a primary closed duodenal perforation, leakage from an oversewn iatrogenic bowel injury, bile leakage, and open rectum stump. In most cases we can only have a fair guess toward the origin of the fistula. During phase I, this is typically all the information required to determine an action plan, as the radiological evaluation is predominantly a CT directed at the detection of a coexisting ongoing infection as an underlying cause of the fistula or as a mechanism that prohibits fistula closure. The old practice of instilling methylene blue via a gastric tube is not very informative and should be abandoned in an era of modern imaging.
During phase II, the stabilization phase (≤10–14 days), focus is on fluid, metabolic and electrolyte balance, adequate nutrition, and wound care. Percutaneous drainage of an intra-abdominal abscess may still play a role in this phase. When untreated infections or non-drained abscesses are no longer an issue, it is time to have a more exact location of the fistula. This is done by “inside-to-outside” contrast imaging and not by fistulogram (“outside-to-inside” contrast imaging). Manipulation in the fistula is not helpful for accurate localization and likely to disturb any potential for spontaneous closure. A fistulogram with water-soluble contrast no longer is considered the “gold standard” for examining a fistula. Inside-to-outside contrast imaging can comprise small bowel contrast radiography with contrast administered via a post-pyloric tube or by mouth, or MR enteroclysis, if the patient’s condition allows. The advantage of MR enteroclysis is that it provides a clear image of the fistula tract as well as the surrounding tissues and any abscess cavity connected to the fistula tract. For initial imaging of a proximal fistula during phase II, contrast radiography usually suffices. In this phase (1) the length of the intestinal tract proximal to the (first) fistula and (2) the length of the fistula tract from intestine to abdominal wall/“outside world” are the primary information needed to determine whether (a) enteral feeding is of any use with respect to absorption length and (b) enteral feeding is feasible with respect to location (e.g., duodenal leak versus fistula of the ileum) and chances of spontaneous closure (e.g., long fistula tract versus fistula in open abdomen). For ICU patients these modalities can be replaced by enteral and intravenous contrast-enhanced CT, but visualization of the fistula anatomy and origin is less clear. For suspicion of a fistula of colonic origin, enteral and intravenous contrast-enhanced CT may be helpful or more feasible and sensitive in this stage than contrast radiography of the colon.
Endoscopy can also be helpful in determining the origin of the disease that caused the fistula, but it is not a particularly helpful or necessary study to reveal a fistula. Biopsy samples could be useful if inflammatory bowel disease, radiation enteritis, or malignancy is suspected. In specific cases with a very proximal, (e.g., esophageal) or very distal (e.g., rectal) origin of a fistula, endoscopy may be useful because of the possibility of endoscopic therapy. Nevertheless, endoscopy should not be considered as a first-step diagnostic tool. Examples of endoscopic fistula therapy are bridging stents in the esophagus or transrectal endoscopic drainage or local vacuum sponge treatment. For gastric and duodenal fistulas, contrast imaging is preferred over endoscopy, as endoscopic fistula therapy does not play a major role in these locations.
Nutritional Support
Key Concept: Adequate nutritional support through both the enteral or parenteral route is paramount for both an attempt of spontaneous closure as well as preparation for operative intervention, if required.
Intestinal failure due to an enterocutaneous fistula is caused by a functional short bowel syndrome. In this situation, the absolute bowel length may be adequate; however, the absorption capacity is only relevant in the intestine proximal from the fistula. The small bowel distal from the fistula is, for all practical purposes, defunctionalized. Therefore, intestinal failure typically presents as malabsorption leading to intractable diarrhea (or output), dehydration (secondary to high-output losses), malnutrition, and weight loss. Total parenteral nutrition (TPN) is the mainstay of therapy for patients with intestinal failure and in particular for those with high-output fistulas, distal obstruction, and ongoing sepsis. TPN maintains good nutritional status and control of fluid, calorie, nitrogen, and electrolyte intake. Moreover, output reduction by TPN reduces wound care problems and risk of dehydration. TPN reduces the maximal secretory capacity of the gastrointestinal tract by 30–50 %. Malnutrition is often a silent, but significant, cause of morbidity and mortality in patients with enterocutaneous fistulas. An additional great advantage of TPN is that it is independent of fistula anatomy.
While the practice of TPN for enterocutaneous fistula has been adopted widely, even for high-output fistulas, additional enteral feeding is beneficial. The primary role of nutritional support, whether enteral or parenteral, is the prevention of malnutrition. Randomized trials investigating outcomes in patients kept “nil by mouth” have not been performed. In fact, it has never been proven that fistula closure rates improve dramatically with TPN compared to enteral nutrition. The concept of “bowel rest” has been based largely on the observation of output reduction, yet output reduction has never been proven to be related to fistula closure [15].
When comparing the oral and intravenous routes, the advantages of enteral nutrition include avoidance of catheter-related complications (sepsis, thrombosis), trophic effect on bowel mucosa, support of immunological and barrier functions of the gut, reduced risk of bacterial translocation, and stimulation of bowel adaptation. Enteral nutrition should be used whenever possible, to some extent, although high-output small bowel fistulas as a rule require supplemental parenteral nutrition to prevent malnutrition and manage output.
Fistuloclysis (i.e., feeding distal to the fistula) is believed to prevent atrophy of the small intestine distal to an enterocutaneous fistula and is performed by placing a feeding catheter into the fistula opening. Some surgeons also believe that subsequent reconstructive surgery is made technically easier, though that statement has never been substantiated. Provided there is more than 75 cm of healthy small intestine available downstream for absorption distal to the fistula opening [16], fistuloclysis makes theoretical sense from a nutritional standpoint and may indeed prevent mucosal atrophy. However, in clinical practice, the enormous effort to provide this fistula feeding often does not outweigh its disadvantages, such as additional wound care difficulties, decreased mobilization of the patient, relatively limited contribution to overall nutrient demand (e.g., feeding the distal ileum has limited nutrient absorption), and unpredictable absorption of nutrients. Therefore, the practitioner must consider the reality that distal feeding does not provide adequate, predictable, and balanced nutrition, whereas TPN combined with proximal enteral feeding does.
Postoperative Nutrition
Key Concept: Intravenous nutrition requirements continue even after definitive surgery due to problems with absorption until adaptation is complete.
It is important to remember that after fistula surgery the downstream part of the intestine has limited function for a prolonged period of time. Although some enteral nutrition is still possible and preferable, resorption of enteral nutrients is not optimal until after the postoperative adaptation phase with intestinal mucosal restoration. Therefore, postoperative TPN is essential to ensure adequate nutrient intake without the pressure of enteral intake to fulfill this need.
Rehabilitation Phase
How to Control Fistula Output and Role of Adjunctive Medications
Key Concept: Several classes of medications are available and often required to reduce fistula output.
The standard regimen to reduce fistula output, if needed, is the use of high-dose antidiarrheals in a combination of loperamide, codeine, and proton pump inhibitors (PPIs). Loperamide acts in the gastrointestinal tract on both cholinergic and noncholinergic mechanisms, thereby decreasing the activity of both longitudinal and circular muscles. Codeine sulfate is an opioid analgesic with weak analgesic properties. Yet its “side effects” include an ability to decrease gastric, biliary, and pancreatic secretions; cause a reduction in motility; and are associated with an increase in tone in the gastric antrum and the duodenum. Digestion in the small intestine is delayed, and propulsive contractions are decreased. Codeine can cause a spasm of the sphincter of Oddi, thereby increasing biliary tract pressure. PPIs are lipophilic weak bases that cross the parietal cell membrane and enter the acidic parietal cell canaliculus. In this acidic environment, the PPI becomes protonated, producing the activated sulfenamide form of the drug that binds covalently with the H+/K+ ATPase enzyme that results in irreversible inhibition of acid secretion by the proton pump. PPIs pantoprazole, esomeprazole, and rabeprazole were more effective in increasing gastric pH and decreasing gastric volume than omeprazole [17]. It is our general practice, after a short period observing natural behavior of the fistula, to start with loperamide, a PPI, and codeine.
Cholestyramine can be added to this regimen, in particular when corrosive action of fistula output creates a wound care problem. Cholestyramine is a chloride salt of a strongly basic non-digestible anion-exchange resin used as a bile salt sequestrant. It binds to bile acids in the intestine to form an insoluble complex, which is excreted in the feces.
It should be especially noted that bulk-forming laxatives are not part of the standard regimen of medical treatment of high-output fistulas. These agents absorb water and cause a softening of stool mass. In addition, the bulk-forming laxatives cause an enlargement of the stools that stimulates propulsive movements in the GI tract and encourages the passage of intestinal contents.
Somatostatin analogues inhibit the release of gastrin, cholecystokinin, secretin, motilin, and other gastrointestinal hormones. This results in a decreased secretion of bicarbonate, water, and pancreatic enzymes into the intestine and an increased water and electrolyte absorption, thereby reducing the intestinal fluid volume. Moreover, analogues relax intestinal smooth muscle, which increases the intestinal capacity. For somatostatin and somatostatin analogues such as octreotide, there is no equivocal evidence that closure rate is improved. In a recent meta-analysis that included randomized trials comparing somatostatin or one of its analogues with control treatment, closure rates are improved by the somatostatin analogues octreotide (5 trials) and lanreotide (1 trial) (RR 1.36 (95 % CI 1.12–1.63); I 2 = 47 %) [18]. This effect is dominated by the results of the two largest trials comprising 192 of 307 pooled patients and including not only 114 small bowel fistulas, but as much as 101 pancreatic and gastric fistulas. The pooled effect of somatostatin is hampered by heterogeneous results (I 2 = 84 %). There is conflicting evidence about its capacity, on top of standard regimen, to reduce output and time to spontaneous closure. We primarily use somatostatin in the setting of high-output fistula despite adequate loperamide, PPIs, and codeine causing fluid and electrolyte disturbances and wound care problems.
Creative Ways for Wound Care
Key Concept: Due to the nature of these wounds, often you are required to think “outside of the box” for innovative solutions to control effluent and protect the skin.
Various solutions can be applied for wound management of high-output fistula. Wound management systems (Fig. 7.3) and negative pressure wound therapy (NPWT) systems with either foam (Fig. 7.4) or gauzes (Fig. 7.5) deliver excellent solutions. In general, wound care in patients with enterocutaneous fistula is tailor-made, and choices must be made per patient, depending on output, localization, abdominal shape, and skin folds. These include wound appliances that can be cut to shape, suction catheters, and adhesive paste dressings to build up or flatten irregularly shaped wounds. When available, often the incorporation of wound care or enterostomal therapist provides additional knowledge and experience that are invaluable to this aspect of care of ECF patients.
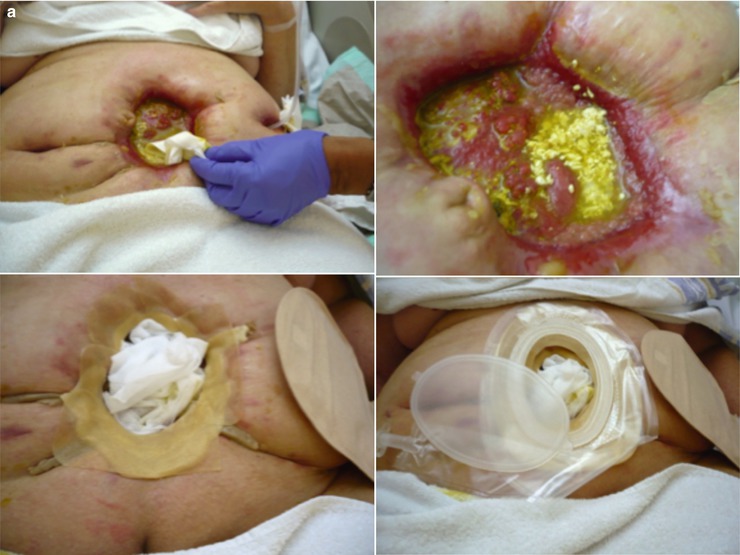
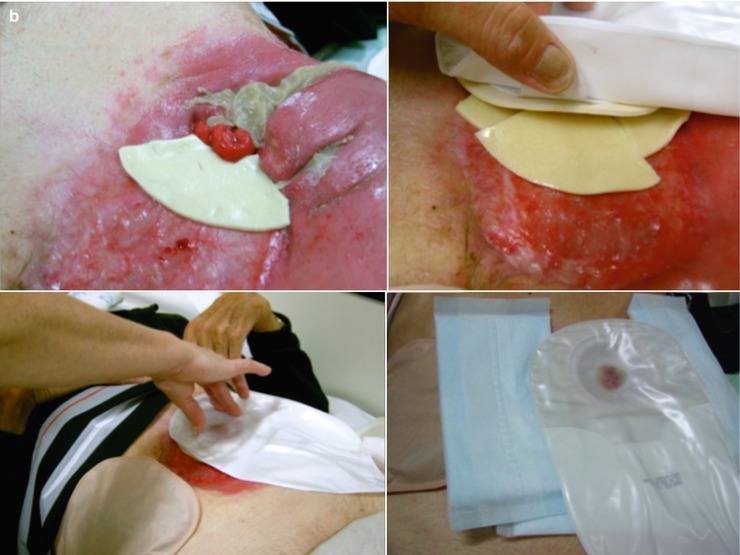
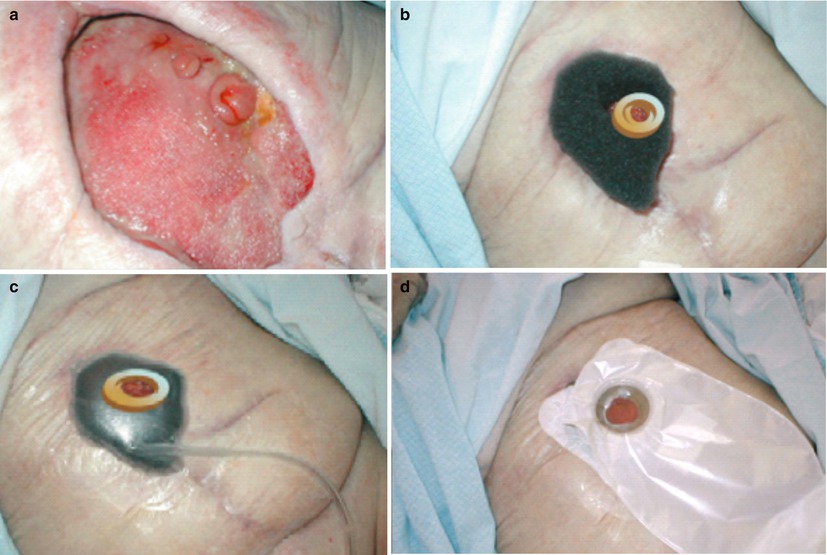
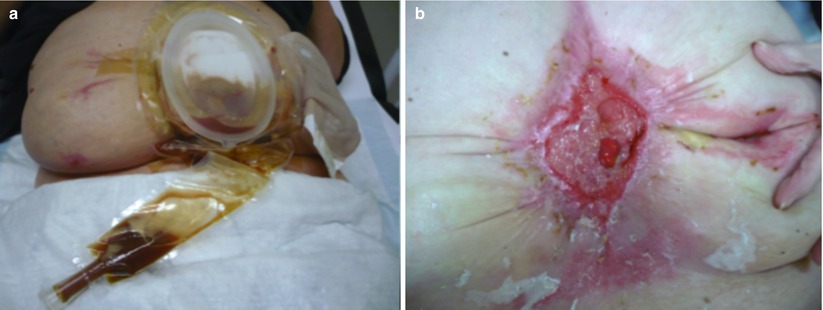
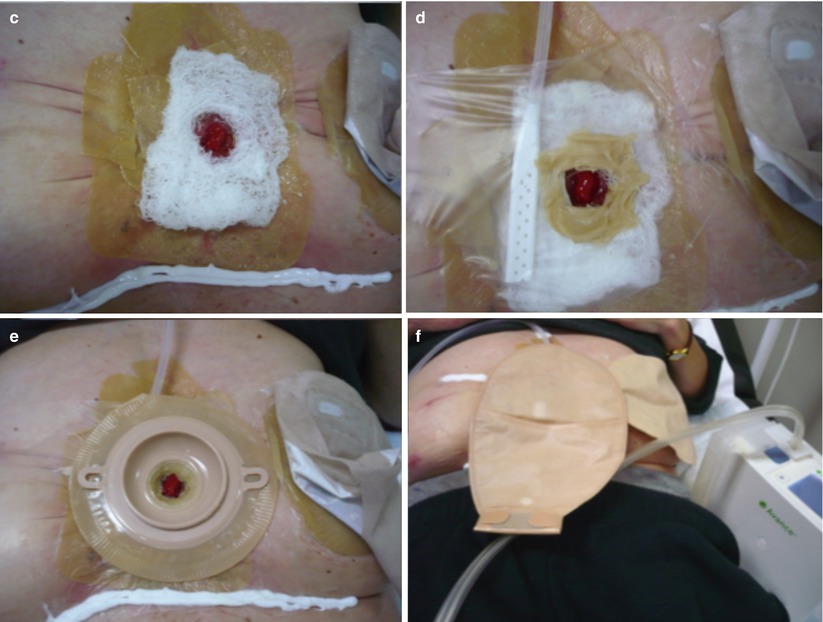


Fig. 7.3
Examples of tailor-made fistula wound care. (a) A deeper laying enterocutaneous fistula with a skin wall. (b) Solution for the (here shown) massive, painful skin erosion caused by fistula effluent (We thank Yvonne Lutgens, specialist nurse, Academic Medical Center (AMC), Amsterdam, for these pictures)

Fig. 7.4
V.A.C. ® negative pressure wound therapy to isolate the fistula. (a) View of granulating open abdomen with fistula. Wound care by V.A.C. ® (KCI) negative pressure wound therapy (NPWT) system to isolate the fistula. (b) Create a donut of petroleum gauze allowing for a clear 1 cm margin around fistula mouth. (c) Invert a stoma ring into a cone to sit inside donut with fistula mouth clearly visible. V.A.C.® GranuFoam™ Dressing to cover wound bed appropriately without overlapping donut. (d) Successful fistula isolation with stoma bag applied on top to collect secretions (We thank Chris Borsten, KCI Medical, Houten, the Netherlands, for these pictures)


Fig. 7.5
Avance ® gauze negative pressure wound therapy to isolate the fistula. (a) Standard wound manager leaks in sitting position, twice a day. (b) View of granulating open abdomen with fistula and clear corrosive injury, on patient’s left side a stoma is seen. (c, d) Wound care was changed to hydrocolloid with (Avance ®, Mölnlycke) gauze negative pressure wound therapy (NPWT) system to isolate the fistula. (e) On top a two-part stoma system. (f) 60 mmHg negative pressure on fistula environment, fistula itself is isolated from negative pressure (We thank Yvonne Lutgens, specialist nurse, Academic Medical Center (AMC), Amsterdam, for the photographs)
Dealing with Medications for Underlying Disease
Key Concept: While fistulas in the setting of active Crohn’s disease may close with immunosuppressive therapy, in general the diseased section of bowel needs to be removed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








