Fig. 8.1
Patient with intra-abdominal sepsis and gut ischemia. While there is no EAF present, they are a prime candidate for this complication
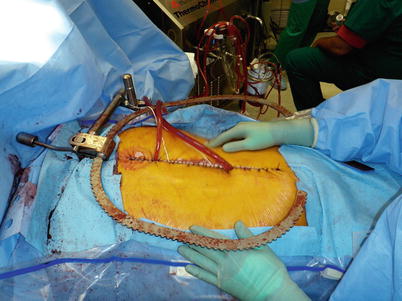
Fig. 8.2
Patient undergoing cytoreduction and HIPEC. These patients are at high risk for dehiscence and subsequent exposure of intra-abdominal viscera
Once an EAF occurs, the patient and surgeon must embark upon what is typically a long journey toward healing. This healing/management process can be arbitrarily broken down into phases of treatment, as has been cited by many authors [13, 14]. Regardless of the specifics of any particular management scheme, they all tend to be based on a few sound tenets: recognition and stabilization, anatomic definition/decision planning, and definitive surgery, if needed [14]. The early phase is characterized first by determining if an EAF is present, followed by early fluid and electrolyte resuscitation, and control of any remaining septic focus. The latter remains an important distinction with EAF patients, where this is often not an issue, as opposed to those with an enterocutaneous fistula. Also in the initial phase, focus is on control of fistula output, protection of surrounding skin, and early nutritional support. The intermediate phase involves defining the fistula anatomy, securing durable access for nutritional support, and planning for the potential of spontaneous closure vs. committing to the long process of definitive surgical management. The late or final phase in management is made up of definitive surgical therapy to close the EAF, reconstruction of the abdominal wall defect that almost always accompanies this process, and prevention of complications related to the closure itself. The remainder of the chapter will address the abovementioned issues with specific attention dedicated to several areas of controversy surrounding the management of EAF.
Prevention
It is important to stress that the best approach to an EAF is to prevent its occurrence altogether (Fig. 8.3). While this disastrous event may be unavoidable, there are factors that increase its risk. Initially it was felt that development of an EAF was increased in patients with an open abdomen for reasons other than trauma; however, a recent report showed this not to be true [15]. Undoubtedly, every attempt should be made to close the open abdomen as soon as possible. While we obviously lack randomized data proving that increased duration of bowel exposure to the outside environment results in an increased rate of EAF formation, this is clearly the consensus [16, 17]. A report published in 2005 reviewing complications experienced in 344 damage control laparotomies showed a higher rate of complications, including EAF, if the abdomen was left open longer than 8 days [18].
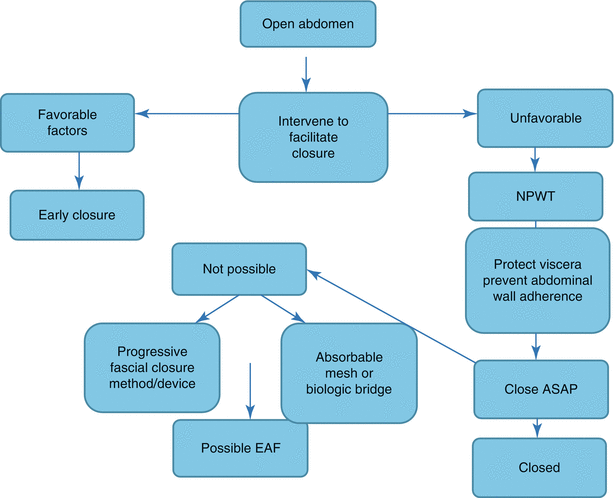

Fig. 8.3
A suggested management scheme for the open abdomen with focus on prevention of EAF. ASAP as soon as possible, EAF enteroatmospheric fistula, NPWT negative-pressure wound therapy
Problem: The Fascia Won’t Close Initially, Now What?
Key Concept: Overlying closure through a variety of techniques is the best way to help prevent EAF formation.
The reality of the situation is that the surgeon cannot simply choose a convenient time to close the abdomen. Typically one has to wait for resolution of visceral edema so that fascial closure can be achieved without leading to intra-abdominal hypertension. There are several reported techniques to potentially reduce the rate of EAF formation in the abdomen left open, and there are also several methods reported to decrease time to closure in these patients. Schecter and colleagues advocate covering the viscera with a non-adherent drape, and performing a skin only closure as an intermediate when fascial re-approximation is not possible [19]. While this seems intuitive, it is based more on expert opinion than any data and may actually result in repetitive trauma to the skin if multiple reoperations are required prior to definitive closure. There are certainly potentially better systems in use today that may hasten fascial closure (Figs. 8.4, 8.5, 8.6, and 8.7).
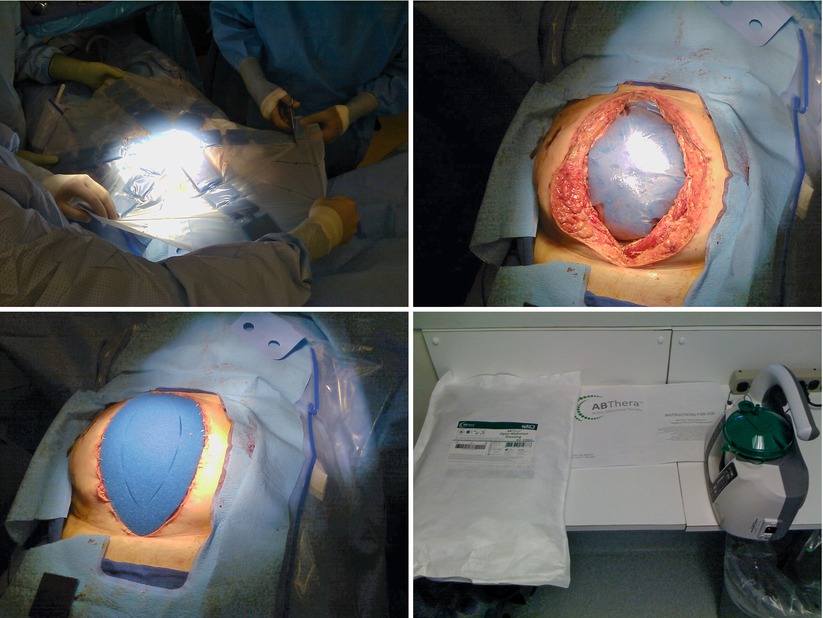

Figs. 8.4, 8.5, 8.6, and 8.7
Use of the VAC ABTheraTM system (KCI, San Antonio, TX). Photos show sizing of the protective drape, placement in the peritoneal cavity, coverage with outer sponge, and the negative-pressure apparatus and fluid collection chamber
The planned ventral hernia (PVH) approach utilizes absorbable polyglactin mesh to create a fascial bridge, effectively covering the bowel. If enough skin is available, it can be closed over drains placed between the absorbable mesh and the skin. This results in a closed peritoneal cavity, but a guaranteed ventral hernia in the future. Although this method was once more popular, it has fallen to a less favored position given the availability of negative-pressure wound therapy, biologic meshes, and other early fascial closure techniques (Fig. 8.8). The use of negative-pressure wound therapy (NPWT) devices in close contact with the bowel is somewhat controversial. Initial success was tempered by fears that this would conversely create EAFs and promote anastomotic leakage. Several more recent reports have either refuted these concerns or have compared NPWT to absorbable mesh closure in patients with an open abdomen, demonstrating superior results in the NPWT group [20–22]. A prospective randomized trial comparing NPWT closure to the use of absorbable mesh in this setting showed a higher rate of fistula formation in the NPWT group (21 % vs. 5 %), but this was not statistically significant given the small number of patients in the trial [23]. NPWT has also been shown to be safe for use in aiding late fascial closure (up to a month after the initial laparotomy) with a low rate of fistulization, allowing avoidance of the PVH approach altogether [24].
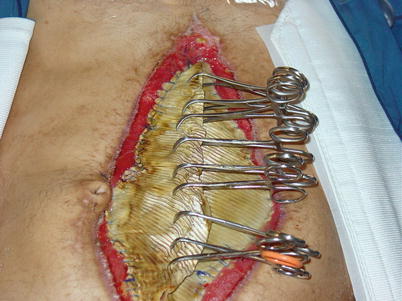

Fig. 8.8
Combat casualty managed with an open abdomen employing coverage of the viscera with PTFE mesh sewn to the fascial edges with progressive tightening at the midline as edema resolves. This is the so-called EDAC (early definitive abdominal closure) technique utilized at Walter Reed Army Medical Center
One issue that can plague any effort to achieve early fascial closure is progressive retraction of the rectus and oblique muscles laterally while the abdomen is left open (Fig. 8.9). Even with reduction in visceral edema, this retraction continues to occur until the linea alba is re-approximated in the midline. While there are many techniques available to prevent abdominal wall retraction, some have been shown in the literature to assist in achieving early (faster) abdominal wall closure [4, 25–28]. The uniting factor involves some type of mesh material fixed to fascial edges, combined with progressive tightening at the midline as visceral edema resolves and the wound is closed (Fig. 8.10). NPWT is employed as an outer wound dressing over the top of the mesh bridge to control fluids and exudate. A key aspect of these techniques is the use of a non-adherent layer or sheet over the viscera inside the peritoneal cavity to prevent adhesions to the anterior abdominal wall resulting in a frozen abdomen.
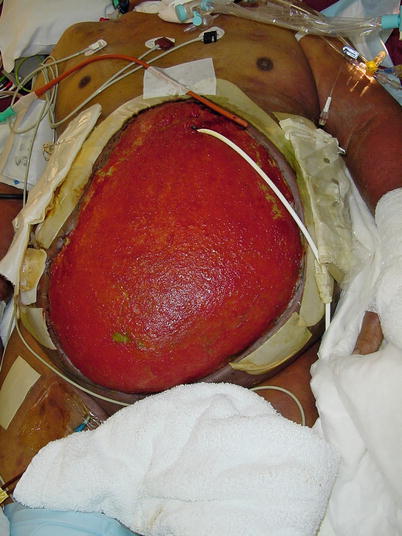

Fig. 8.9
A patient managed with an open abdomen after a repair of a ruptured abdominal aortic aneurysm. This patient was managed in the pre-NPWT days, and the viscera are covered with a healthy bed of granulation tissue. A Foley catheter has been placed in the stomach for feeding purposes

Fig. 8.10
EDAC patient after closure of the fascia primarily at the midline
In cases where several days have passed and early closure seems impractical, one may choose to use biologic mesh bridges to achieve fascial “closure” with either skin re-approximation over drains or NPWT over top of the biologic graft (Fig. 8.11). While this has been shown to result in a high rate of incisional hernia formation [29, 30], it achieves the goal of skin closure over the viscera and has been shown to result in a low rate of bowel fistulization [31]. While some believe that placement of a biologic bridge results almost universally in an incisional hernia over the long term, others have shown that this complication can be minimized (33 % vs. 83 %) if skin closure over the biologic bridge can be achieved immediately [32]. Follow-up in this particular study was short (9 months), limiting the generalizability of the conclusions. Early closure using a variation of the component separation technique (CST) can be performed and has been shown to potentially eliminate the risk of fistulization [33]. However, one must consider the risk of eliminating future options for abdominal wall reconstruction should CST failure occur. The first effort with CST is usually the best and potentially the only chance to achieve a desirable result.
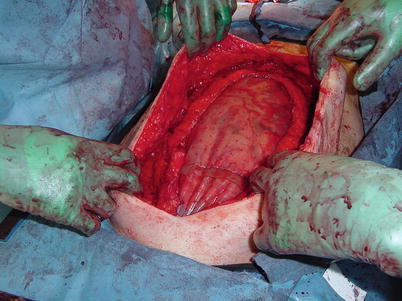

Fig. 8.11
A large fascial diastasis that has been bridged with a biologic mesh. While this is not optimal in terms of hernia repair, it may be an acceptable option to obtain visceral coverage
An Ounce of Prevention
Key Concept: Avoiding serosal tears in dressing changes and early nutritional support in the open abdomen setting helps reduce EAF formation.
It is imperative that an experienced member of the surgical team be present during dressing changes for the patient with an open laparotomy wound. This can ensure the avoidance of trauma to the underlying viscera as well as early recognition of areas of deserosalization that are likely precursors of an EAF. Girard reported securing of human acellular dermal matrix (HADM) sheets to areas of intestinal deserosalization with fibrin glue [34]. This was performed in two patients felt to be at risk for EAF, which ultimately did not occur in either. The use of this method has also been reported to be successful in closing small EAFs [35, 36].
While nutritional optimization is central to the care of a patient with a gastrointestinal fistula, it is almost as important in the prevention of an EAF. A patient with an open abdomen is in an extreme catabolic state with increased nutritional requirements. The benefits of enteral nutrition over parenteral nutrition are well established in surgical patients, and the use of early (less than or equal to 4 days after laparotomy) enteral nutrition has been shown to result in a statistically significant reduction in the rate of EAF formation, as well as a faster time to abdominal closure [37]. While no single strategy will prevent EAF in every patient managed with an open abdomen, the above mentioned tools may be tailored into the care for these patients to minimize the risk of this devastating complication.
Problem: So You Have an EAF (The Early Phase)
Key Concept: In the early phase, the goals are to categorize the EAF as superficial or deep to help define an uncontrolled infection, followed by implementation of sepsis control, nutritional therapy, and skin protection measures. Success (or failure) with each of these components has an interrelated effect on optimizing the others.
Diagnosis
Unfortunately, despite our best preventive efforts, some patients will develop an enteroatmospheric fistula (Fig. 8.12). Recognition of this complication is not typically difficult, as one will appreciate either stool or bilious material draining from the open wound (Fig. 8.13). Once these signs are recognized, one must determine if the EAF is deep or superficial. A superficial fistula is easy to see during physical examination of the patient. Often a clearly visible enterotomy or colotomy (Fig. 8.14) will be noted, but in many cases, definitive recognition of the source may be more difficult. Irrigation of the wound bed followed by focus on the region of the wound that drainage appears to emanate from will often lead to localization of a pinhole enterotomy. Once a superficial source is confirmed, focus can turn to early supportive care of the patient.
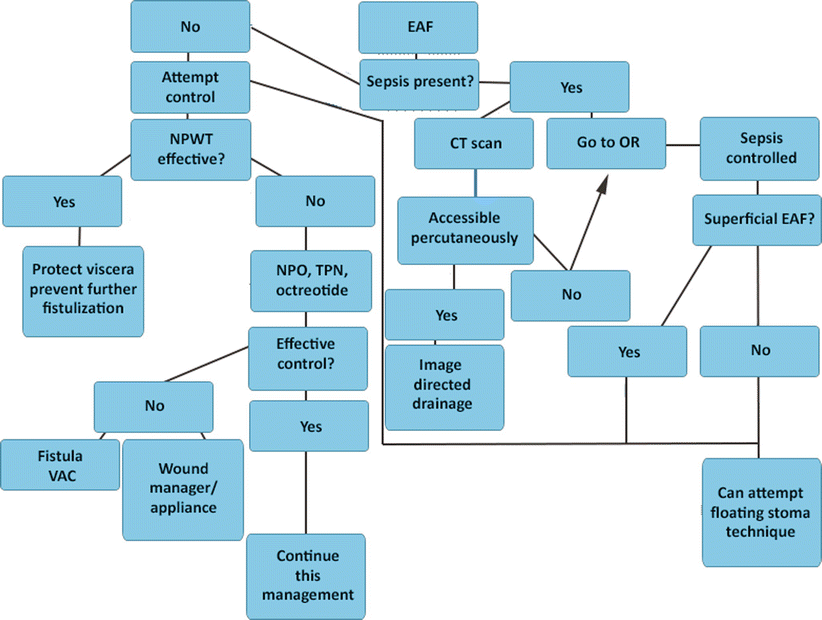
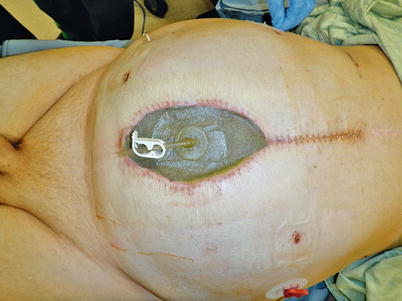
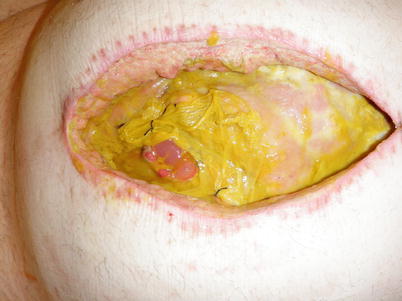

Fig. 8.12
Algorithm depicting methods useful in managing an enteroatmospheric fistula after it is initially discovered. EAF enteroatmospheric fistula, NPWT negative-pressure wound therapy, OR operating room, NPO nothing per os, TPN total parenteral nutrition, VAC vacuum-assisted closure device

Fig. 8.13
Image showing a NPWT system that has been overwhelmed by a high-output EAF. This dressing was placed less than 1 day prior to this photo

Fig. 8.14
A superficial coloatmospheric fistula
Superficial vs. Deep
Differentiation between superficial or deep EAF is important simply from the standpoint of uncontrolled sepsis. The first step in the early phase after recognition is control of any septic focus. While the presence of an undrained abscess is uncommon in the patient with an EAF, it is more common in an individual with a deep fistula source. The typical scenario is an anastomotic leakage in a patient being managed with an open abdomen. If the site of anastomosis lies deep within the peritoneal cavity, undrained collections may be present despite the fact that some drainage is noted in the open wound. Computed tomographic scanning is the best method to demonstrate any intraperitoneal collection needing attention. A patient showing systemic signs of sepsis should be treated with broad-spectrum antibiotics and undergo drainage of any septic collection. In some cases this can be performed percutaneously using CT guidance; however, in others this is not possible due to lack of an intervening safe window of passage for needle and drain. It is this group of individuals that may require early reoperation simply for control of sepsis.
In a small but fortunate subset of these individuals, the problem of EAF may be addressed definitively at the re-exploration through resection of the leaking segment of bowel with reanastomosis, proximal diversion, or both depending on the individual setting and sound surgical judgment. When considering the decision to perform an anastomosis in this setting, we must account for the fact that the patient will likely continue to require management with an open abdomen and that they initially developed the EAF/leak in this very environment. The expectation that a new anastomosis will heal in a worse environment is unlikely, outside of the rare event of discovering an isolated identifiable and modifiable factor that led to the leak in the first place. Consideration of early proximal diversion in this situation followed by the previously mentioned methods to prevent re-fistulization may shorten the course of this complication dramatically. Sadly this is not often possible, and even when attempted, the result is often EAF recurrence. There are also instances where proximal diversion is desired but impossible due to mesenteric foreshortening and bowel immobility. In cases such as this, the only option available may be to widely drain a leak with multiple closed suction or sump-type drains. Elimination of oral/enteral intake may be required to achieve effective control of drainage.
Control of Sepsis and Resuscitation
Once it is recognized that there will be no rapid solution to the problem of EAF, the surgeon and patient will embark upon the long journey involved in treating this process. The early phase of management that remains involves resuscitation, nutritional support, control of output, protection of surrounding skin, and creation of a plan for the subacute and chronic management of this problem. Resuscitation occurs simultaneously with diagnosis and control of sepsis. In the earliest phase, it is often sepsis that drives resuscitative needs, though a high-output fistula (>500 mL/day) will lead to substantial fluid and electrolyte loss requiring replacement. The type of fluid required for replacement therapy is dictated by the site of fistula origin, with normal saline + 10 mEq/L KCL being effective for most EAFs. Very proximal small bowel or duodenal fistulas may require bicarbonate replacement as well. Until the situation begins to stabilize, frequent analysis of serum electrolytes will be required to correct any necessary deficiencies. Basic principles of fluid resuscitation apply to these patients as they would to any postsurgical patient.
Early Nutrition
Sound and effective nutritional support is central to the care of the EAF patient either to provide the highest chance of nonoperative closure or to optimize the patient for eventual surgery (Fig. 8.15). The question of enteral vs. parenteral nutrition is always simple for the nutritionist—with enteral almost always being the preferred route—but is much more complex for patient, surgeon, and nurse caring for the patient. Because of difficulty with control of effluent, enteral nutrition is rarely an option in the early phase of EAF management. Even a “low”-output fistula may not be suitable for the enteral route of replacement, as they will often convert to a high-output fistula when the gut is used for feeding. Parenteral nutrition via a central venous catheter will likely be the best option in this phase. With the majority of these patients in a profoundly catabolic phase, the standard postoperative nutritional recommendations of 20 nonprotein kcal/kg and 1.5 g/kg of protein may not be sufficient. The patient may require up to 30 nonprotein kcal/kg and 2.5 g/kg of protein with supplementation of zinc, vitamins, trace elements, and five to ten times the standard recommendation of vitamin C [13]. Additional copper, folic acid, and vitamin B12 are often also needed [38].
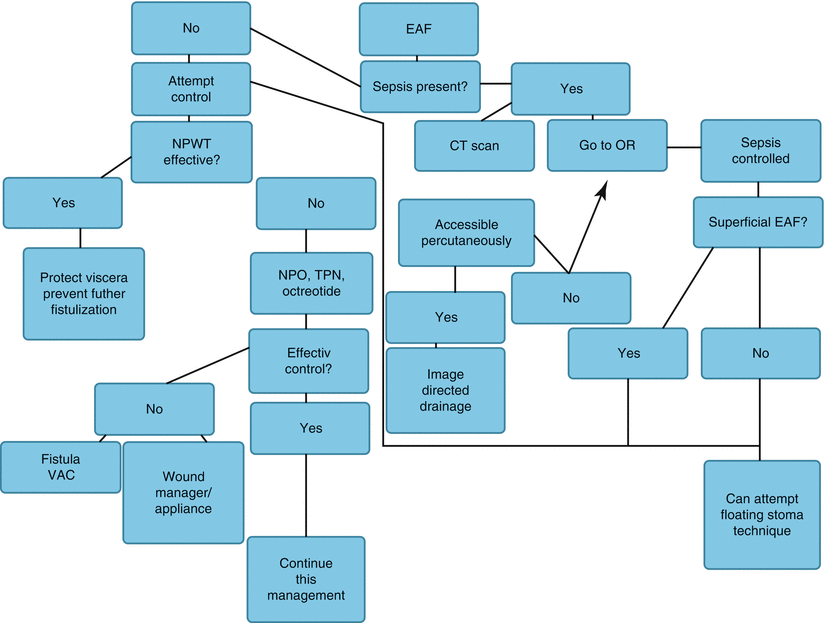

Fig. 8.15
Algorithm illustrating a feeding strategy to be used early after discovery of an EAF. EAF enteroatmospheric fistula, NPO nothing per os, TPN total parenteral nutrition, VAC vacuum-assisted closure device
Assessment of nutritional adequacy should also begin in the early phase with twice weekly measurements of serum prealbumin. The serum C-reactive protein level is also often helpful in determining whether or not the patient remains in the acute inflammatory phase or has ongoing sepsis. No matter how much nutritional support is provided, it is difficult to make measurable gains in the nutritional status of an actively septic individual. This again illustrates the importance of sepsis control in optimizing a patient’s chance to heal an EAF nonoperatively or at least provide favorable conditions for a future required surgical procedure.
Effluent Control and Skin Protection
It is worth repeating that the poorly controlled EAF is a nightmare for the patient and everyone involved in their care. It is a source of embarrassment and discomfort for the patient, frustration for the surgeon, and results in the consumption of a tremendous amount of nursing and disposable medical resources. Early control of EAF output is therefore critical, as contact between the skin and drainage will result in significant skin damage that may limit options for subsequent control. A sound first step is to stop any and all oral intake. Bowel rest will likely not eliminate EAF output, but will significantly reduce the quantity. Use of a nasogastric tube on intermittent suction may also aid in reducing the quantity of effluent. In the majority of cases, the use of NPWT will have already been employed, and simple continuation of this will be all that is needed to obtain early effluent control. EAFs that result in higher effluent output will often overwhelm NPWT systems resulting in the requirement for dressing changes on a daily basis or even more frequently. This again can overwhelm both manpower and resources requiring advanced methods of control (Figs. 8.16, 8.17, and 8.18). The involvement of an enterostomal therapist or experienced wound care team cannot be overemphasized [39]. If the patient is being cared for in a facility without these resources, transfer to a higher level of care should certainly be considered.
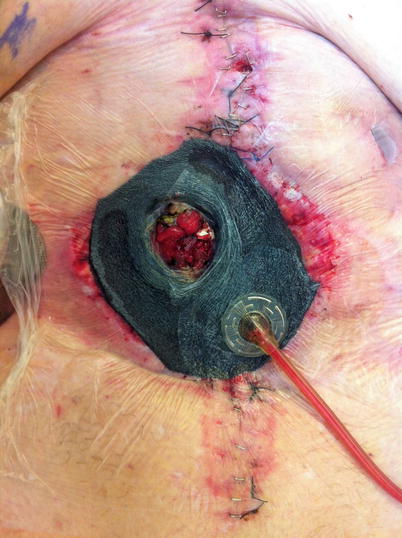
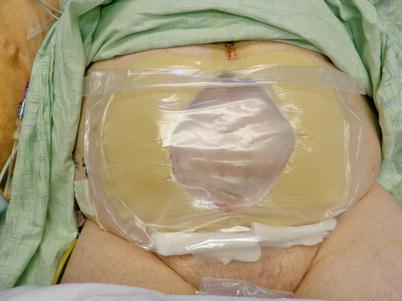
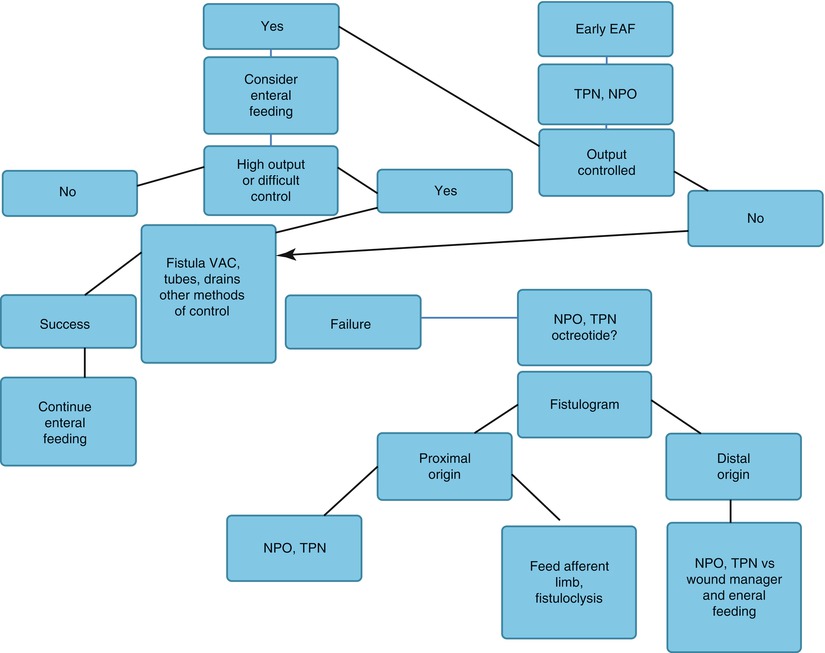

Fig. 8.16
The “fistula VAC.” The EAF has been isolated from the rest of the wound bed allowing the benefits of NPWT while controlling effluent using and ostomy appliance

Fig. 8.17
Use of a large custom fit wound appliance. This technique is useful when NPWT fails to adequately control fistula effluent

Fig. 8.18
Use of baby bottle nipples with Malecot drains inserted in them to isolate two EAFs from the wound bed so NPWT could be used
There are several available options for skin protection using any of a variety of topical skin barriers. Again, the enterostomal therapist/wound care team will be familiar with the available options, and use of these materials should be employed early. Advanced method of effluent management as well as pharmacologic adjuncts to the management of EAF will be discussed in the next section.
Intermediate Phase
Key Concept: Once the patient has stabilized, the focus shifts to using enteral nutrition, defining the fistula anatomy, identifying potential sources hindering EAF resolution, and mobilization or discharge of the patient through advanced wound protection. This may allow the EAF to close spontaneously or prepare the patient for surgery.
The intermediate phase in the care of the EAF patient is characterized by anatomical definition of the fistula, obtaining durable feeding access or employing alternate feeding strategies, use of advanced wound care and control techniques, and tailored management toward defined goals of spontaneous closure vs. future surgical closure. Psychiatric and social support of the individual with EAF cannot be overstated. It is this phase of management where the patient, their family, and nursing will apply pressure to the surgeon in hope of a quick fix. Unfortunately, there is no easy way out of this complication, and patience with well-defined goal-directed management must be employed. Surgeons must resist pressure to attempt surgical intervention too soon, as the error of early surgical intervention often results in secondary complications potentially worse than the original problem.
Defining Anatomy
Definition of EAF anatomy can be helpful in determining prognosis, in preoperative planning, and in creating a feeding strategy. While it may be obvious whether an EAF is of colonic or small bowel origin on simple inspection of the wound bed, in some cases (especially with deep EAF), this distinction is difficult to make. Fistulas that originate more distally in the small bowel or those from the colon are often more likely to close spontaneously. Deep EAFs are also more likely to close, as long as sepsis is controlled, given the length of the fistula tract. While these statements are difficult to support directly with evidence, one can extrapolate from evidence that reveals lower spontaneous closure rates in fistulas with proximal origin or high output [40]. There is also some evidence to suggest that ECFs that have developed in trauma patients may be more likely to close than in others; however, it is unknown if this association applies to those with an EAF [41]. Knowledge of the likelihood of spontaneous closure will affect the goal-directed management plan as well as provide reasonable expectations for the patient and others involved in their care. Site of origin may also directly impact upon the decision to feed enterally or parenterally.
We are often required to perform some sort of radiographic study to make a definitive determination of the site of fistula origin. There are several options available to the surgeon for this purpose. The fistulogram is the classic study to assist in this purpose. Performance of a fistulogram involves intubation of the fistula tract from the outside using various tubing or catheter devices that will facilitate direct contrast instillation into the tract. A scout radiograph should be obtained prior to any contrast administration. This will assist in visualizing any clips or anastomotic staples in the area of question that may be related to the process of fistulization. Contrast should be administered gently under low pressure while visualizing the area of interest using fluoroscopy. The use of a balloon tipped catheter inflated with low pressure may assist in maintaining intraluminal contrast thereby improving the image. A well-performed fistulogram of a deep EAF will define the tract, its length, the origin, and any associated abscess cavity. Fistulograms performed on superficial EAFs may assist in determination of how proximal or distal the origin of the fistula is in the GI tract. Water-soluble contrast should be used when performing these studies. While barium tends to provide greater detail, water-soluble contrast material works well and alleviates the risk of peritonitis related to barium extravasation in some circumstances. Any barium retained in the GI tract tends to form concretions that can be very difficult to clear and may hinder further radiographic evaluations. As a general rule, barium is simply best avoided in these patients. A fistulogram may also aid in the identification of distal obstruction as well as adjacent or associated foreign bodies—both factors that will tend to preclude spontaneous fistula closure. Alternatively, a small bowel follow-through after ingestion of oral water-soluble contrast may also be helpful in identifying the discussed findings.
Computed tomography scanning is probably the most useful modality for imaging a patient with an EAF to determine if there are other associated intraperitoneal abnormalities. When oral and IV contrast are used, one may in fact achieve direct visualization of the origin of the EAF, as well as information related to tract length, associated abscess, adjacent inflammation, presence and relation of foreign body, and any potential distal obstruction. Cross-sectional imaging with CT also provides the obvious advantage of facilitating percutaneous drainage of intra-abdominal fluid collections. MRI and ultrasound examination may also be helpful in select circumstances but are less frequently employed methods of imaging in these patients.
Nutrition
As mentioned, the purpose of imaging is to define the situation anatomically such that an intervention can be undertaken to improve outcome, if possible. Intervention may be direct, as in the case of abscess drainage, or indirect such as implementation of an enteral feeding plan based on anatomy. In cases where fistulas are located distally, it may be possible to provide all nutritional intake by mouth without substantially increasing fistula output to a level where control becomes difficult. In cases where the fistula origin is very proximal, enteral feeding can often be achieved via a tube inserted into the efferent portion of the tract. The majority of intestinal absorptive surface can be utilized, and high volume biliopancreatic secretions can be re-fed into the distal bowel [13]. Some patients will simply not be candidates for enteral nutrition (although it should always be the first choice) and will require extended administration of total parenteral nutrition (TPN). These individuals will require durable central venous access in some form. Line sepsis and TPN-associated liver disease remain major morbidities in those who require this form of nutritional therapy. While debatable, when fistula anatomy is favorable for a higher likelihood of spontaneous closure, some physicians prefer the parenteral route alone in order to keep effluent output low. If spontaneous closure seems unlikely, which is often the case, feeding enterally may be the best way to boost nutritional status in preparation for future surgery.
Effluent Control and Skin Protection
In the intermediate phase, control of fistula effluent remains extremely difficult. As patients regain strength, simple mobilization and, eventually, life beyond the hospital become a reality. This poses challenges to the effluent control aspect of care. In order to re-feed biliopancreatic secretions, they must be effectively controlled and collected. While possible through use of nasogastric tubes and NPWT systems, this is a much more difficult task in practice. High-output EAFs will tend to overwhelm NPWT systems requiring extremely frequent dressing changes and resulting in high cost. For those without as much experience in dealing with this situation, it should be highlighted that an EAF that arises in the setting of the open abdomen often actually gets worse before it starts to get better (Figs. 8.19, 8.20, 8.21, and 8.22). Control of effluent and protection of surrounding skin present a true challenge in this period. Poor control of gastrointestinal secretions will lead to a frustrated patient and nursing staff.
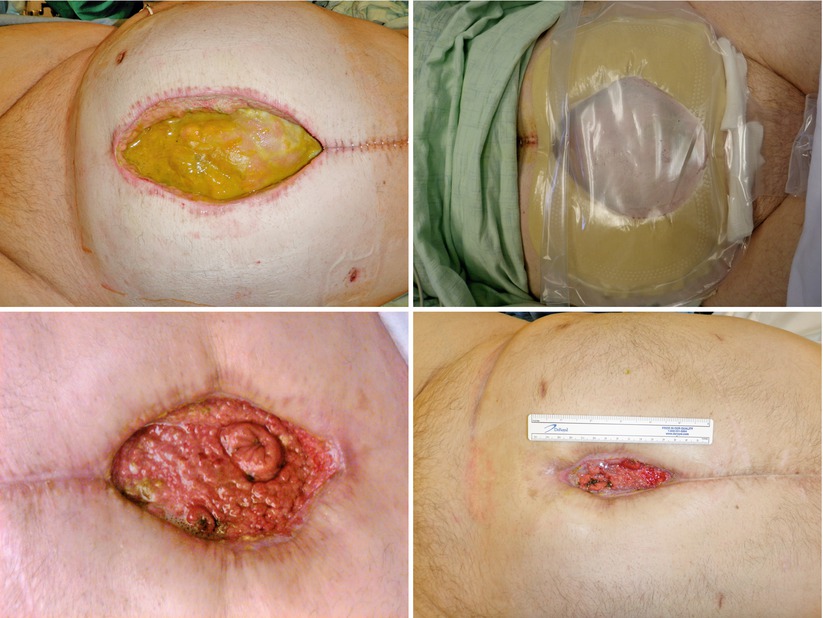

Figs. 8.19, 8.20, 8.21, and 8.22
This series of images shows an EAF patient as they progress through the phases of management. The first image shows a large poorly controlled EAF associated with a wound that actually gets wider initially and is controlled with a wound appliance. Over time the wound bed granulates and contracts ultimately leaving the patient with a very small open wound with two mature fistulas
Aggressive effort toward the above goals is warranted immediately and may require considerable thought. Several authors have developed methods and systems, simply out of need, to address these concerns [42–48]. Creation of a “floating stoma” has even been reported and may be useful in specific circumstances [49]. All of these methods address a few simple ideas: “dam off” the EAF from surrounding bowel or granulation tissue, provide NPWT to surrounding tissues to assist with healing and exudate control, protect the surrounding skin to assist with dressing adherence and use in future surgery, and prevention of trauma to underlying viscera to eliminate the potential for additional EAF formation (Figs. 8.23, 8.24, 8.25, and 8.26). Any system that can address all of these concerns will be effective, but none specifically designed for the purpose of EAF control has been marketed. It therefore requires considerable effort from the care team to design a custom device for a particular patient and to ensure its effective use on a daily basis. This is where a competent enterostomal therapist or wound care team is worth their weight in gold.
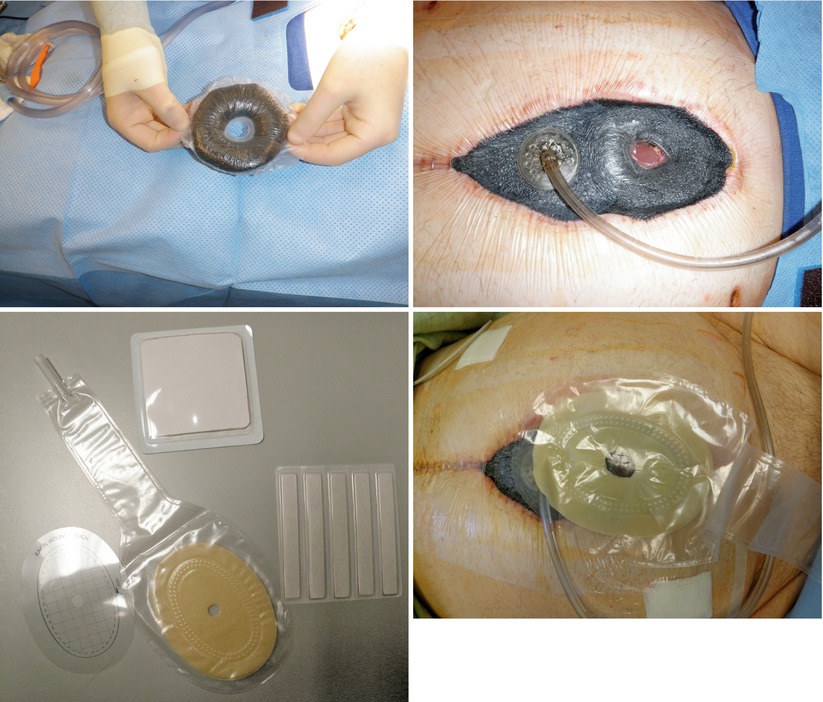

Figs. 8.23, 8.24, 8.25, and 8.26
This series of images depicts construction of a “fistula VAC.” A “donut” is constructed out of sponge and nonporous drape. This is utilized to dam off the EAF from the surrounding wound. Stoma paste and powder can be used to facilitate this. The remaining wound bed is covered with sponge and drape. A fistula appliance is placed over the EAF to control effluent
In cases where effluent control is simply impossible with NPWT-based wound care systems, the only remaining option may be the use of what amounts to a large stoma appliance or wound manager (Fig. 8.27) [48]. These devices can be custom cut to the size and shape of the open wound and function much like a standard ostomy appliance. They come in a variety of sizes and are marketed by at least two companies currently. If the surrounding skin is in good shape, a watertight seal can be maintained with effective collection of effluent in a large pouch. Despite continued contact with gastrointestinal secretions, granulation tissue will somewhat surprisingly continue to form over the underlying viscera, and the wound will contract over time (Figs. 8.28, 8.29, 8.30, 8.31, and 8.32). The wound appliance should be replaced with a fresh one as needed or ideally every 4–5 days, much like an ostomy appliance is managed. As a general rule, changes should be as infrequent as possible to limit trauma to the underlying skin.
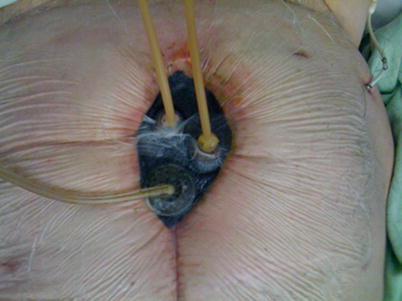
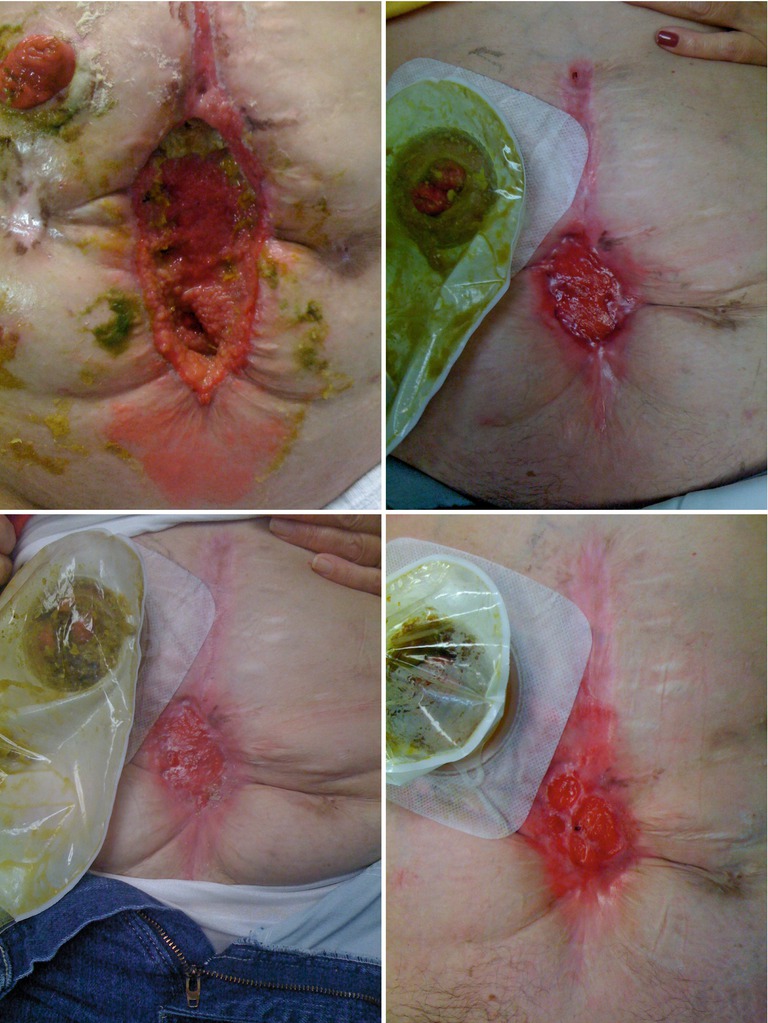
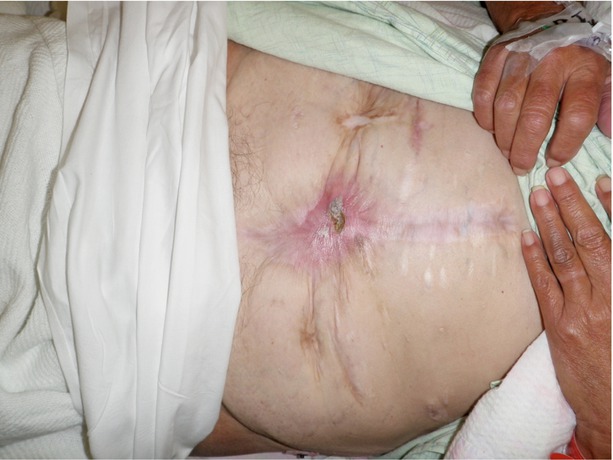

Fig. 8.27
Wound appliance in place


Figs. 8.28, 8.29, 8.30, 8.31, and 8.32
This series of images shows a poorly controlled deep EAF with resulting skin irritation and an adjacent loop ileostomy. This individual was managed with a wound appliance, and despite chronic contact of the wound bed with fistula effluent, the wound contracted nicely over time with resultant spontaneous closure of the deep EAF. The ostomy was taken down and the patient did well
Pharmacologic Therapy
Key Concept: Several pharmacological agents including octreotide, somatostatin, acid–reducing medications, and antimotility drugs aid in decreasing EAF effluent volume and ultimately help in EAF control, volume and electrolyte abnormalities, wound care, and closure.
Ultimately, the goal remains for either spontaneous EAF closure, provision of an acceptable wound bed for performance of split-thickness skin grafting (STSG), or optimization of the overall situation in preparation for future surgery. Optimal control of fistula output is key to achieving these goals but, as stated, can often be difficult in execution. In this case, pharmacologic adjuncts assist in reduction of fistula output and aid in EAF effluent control. The most widely utilized and studied adjunct is octreotide. Octreotide is the long-acting synthetic analogue of somatostatin, a naturally occurring hormone that reduces gastrointestinal, biliary, and pancreatic secretions while increasing intestinal electrolyte and water absorption [50]. These effects are understandably beneficial in the patient with an EAF. Though often not stated, the drug also has the potentially negative effects of decreasing the release of growth hormone and thyroid stimulating hormone [51].
The role of octreotide in the management of EAF has not been specifically investigated; however, numerous studies of the drug’s use in patients with enterocutaneous fistulas exist [50, 52–61]. Claims that octreotide and somatostatin reduce fistula output and result in a higher rate of spontaneous fistula closure are controversial. A 2011 meta-analysis of the role of these drugs in patients with enterocutaneous fistulas concluded that both drugs shorten the time to fistula closure, but only somatostatin improved the rate of spontaneous closure [52]. There are several analyses that show a reduction in fistula output with the use of these medications [55–57, 59, 61], while others show questionable or no benefit [50, 53, 60]. A single center study of 60 ECF patients showed no benefit to the use of octreotide, though did show an increase in septic and thrombotic complications in those in which the drug was used [58]. Another randomized controlled trial of the use of these drugs in 2004 showed that both somatostatin and octreotide reduced time to ECF closure as well as overall hospital costs [54]. Given the mixed nature of reports in the literature, the use of these pharmacologic adjuncts should be individualized in EAF patients, keeping in mind the potential negative effects and complications associated with their use. Drugs such as proton-pump inhibitors and histamine-2 receptor blockers have been shown to be beneficial in patients with short-gut syndrome [62]. The benefit is at least in part related to a reduction in upper gastrointestinal secretions. While most EAF patients will be receiving one of these drugs given the nature of their disease, there may be a benefit, although unproven, in reducing EAF output. Patients with more distal fistulas may benefit from the use of loperamide to control effluent, although there is no data to support this drug’s use for this purpose.
Psychiatric Implications of EAF
Key Concept: The psychiatric toll on both the patient and surgeon is tremendous. Open discussion between all parties, including airing of frustrations, anger, or concerns, is beneficial. Managing expectations and the involvement of mental health specialists can aid in this process.
Dealing with an EAF presents numerous challenges to the patient and their family. There are significant psychiatric implications associated with this disease process. Unfortunately, EAF is a problem with no rapid solution. In the best cases a fistula may close nonoperatively after a significant amount of time (i.e., months) and intensive care management. Patient activity is typically severely limited during the early and intermediate phases of disease. Difficulties with effluent control can prevent a patient from ambulating any significant distance and may completely eliminate the ability to perform normal activities of daily living. Problems with body image occur as a direct result of the physical appearance of the abdomen, the smell of the EAF effluent, and the potential or actual leakage around wound appliances that can be devastating for a patient’s psyche. Even if a patient becomes stable enough to leave the hospital, they often become homebound and reclusive, with limited mobility, while they await spontaneous closure or corrective surgery. Because of this, patients and family will often pressure the surgical team for a quick solution. While no published data addresses the issue in EAF patients specifically, depression is clearly a major problem faced by these individuals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








