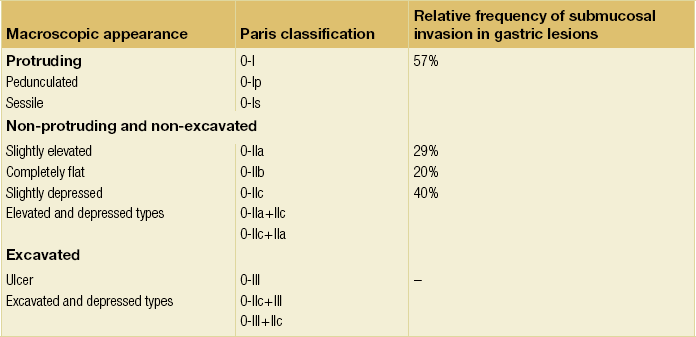
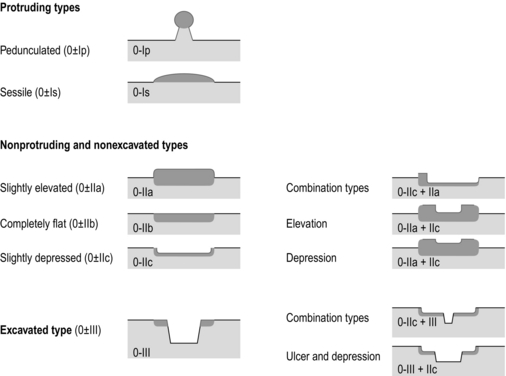
8 Definition of early gastric cancer Early gastric cancer (EGC) is defined as a tumour that is limited to the mucosal or submucosal layers (T1 cancer) and by definition absence of invasion into the proper muscle layer. This is irrespective of the presence of lymph node metastasis.1 The incidence of gastric cancer has steadily declined over many decades, yet it remains worldwide one of the most common malignancies. Most gastric cancers arise as a result of lifelong colonisation with Helicobacter pylori, inducing chronic active gastritis. An abundance of research over the past 20 years has yielded endoscopic and non-invasive methods to recognise both this infection and the various stages of the cascade leading from chronic gastritis via atrophic gastritis, intestinal metaplasia and dysplasia to early and advanced gastric cancer. Cohort studies with these methods have revealed the cancer risks associated with each premalignant condition, and showed that these risks increase with each subsequent stage of the cascade.2 These advances have led to common identification of patients with dysplasia and early cancer of the stomach, a development which likely will be further enhanced by the recent introduction of a guideline for surveillance of patients with intestinal metaplasia and dysplasia of the stomach,3 in particular when present in both antrum and corpus.4 This chapter deals with the management of early gastric cancer. Early gastric cancer usually arises in a mucosa that has undergone atrophic and metaplastic changes. These are recognisable with modern high-definition endoscopic equipment, in particular when combined with additional image enhancement techniques such as narrow-band imaging (NBI). Against this background, most EGCs amenable for endoscopic resection can be recognised by experienced endoscopists.5 Complete staging and classification of EGCs is usually done in three steps. The first step is a pre-interventional endoscopic staging determining depth of tumour infiltration (with or without endoscopic ultrasonography) combined with histopathological sampling. The second step is the actual endoscopic resection, where the most valuable information is gathered from the submucosal lifting properties of the lesions. The third step is the final histopathological staging of the resected lesion. Experienced endoscopists performing endoscopic mucosal resections (EMRs) or endoscopic submucosal dissections (ESDs) are trained to recognise and delineate early neoplasia, which includes assessment of invasion depth and thus the endoscopic resectability of the lesion. EGCs are classified by their endoscopic appearance according to the Paris classification (Fig. 8.1).1 Superficial lesions are classified as either protruding (Paris 0-I), elevated (0-IIa), depressed (0-IIc) or excavated and often ulcerated lesions (0-III). Lesions that have both elevated and depressed components are classified into two groups: depressed lesions in which most of the surface is depressed and there is elevation in a portion of the peripheral ring are classified as 0-IIc + IIa, while elevated lesions with a central depression encircled by the elevated ring at the periphery are called 0-IIa + IIc. The combined patterns of excavation and depression are called 0-III + IIc or 0-IIc + III, depending on the relative surface area of the ulcer and of the depressed area. The classification helps to predict the extent of invasion into the submucosal layer and thus the choice between endoscopic or surgical treatment (Table 8.1). For instance, true protruding lesions in the stomach demonstrate a 57% relative frequency of submucosal invasion, whereas non-protruding, non-excavated lesions demonstrate submucosal invasion in frequencies between 20% and 40%. In excavated lesions, often the proper muscle layer is already involved. Although the exact percentage of involvement of the proper muscle layer is not reported in the literature, this number comes close to 100% and the Paris 0-III excavated types of lesions are usually not resectable by endoscopic means. Additional characteristics that predict submucosal or deeper invasion are larger tumour size (> 30 mm), presence of discoloration (remarkable redness) and ulceration.5 Lesions that are confined to the mucosa tend to move over the peristaltic waves, whereas peristaltic waves seem to curve around tumours that have invaded the proper muscle layer. The latter provides a strong argument against endoscopic resection. In most EMR and ESD techniques, submucosal injection of fluid is used to lift the early cancer from the proper muscle layer. This method has three major benefits: (i) it provides information on invasion depth and thus endoscopic resectability; (ii) it facilitates endoscopic resection; and (iii) it provides a safety fluid cushion for resecting the superficial lesion without damaging the deeper layers when using snares, knives or electrocautery.6 The amount of lifting provides information on the invasion depth of the lesion. Mucosal or superficial submucosal lesions usually demonstrate complete lifting (m–sm1), whereas the lesions that infiltrate into the deeper submucosal layers often lift incompletely (sm2–sm3). A non-lifting sign most often represents invasion deeper than sm3.6 Endoscopic staging before resection does provide useful information on the prediction of lymph node metastasis. Differentiation grade can be assessed through simple biopsy. A large retrospective study from Japan assessed the occurrence of lymph node metastasis in 5265 patients who had undergone gastrectomy with lymph node dissection for early gastric cancer.7 All specimens were reassessed for macroscopic appearance, size, ulceration, invasion depth and extent of submucosal invasion, differentiation grade and lymphovascular involvement. The results are summarised in Table 8.2 and define the criteria for EMR and ESD.7,8 The role of endosonography remains debatable in EGCs. Endosonography lacks sufficient diagnostic accuracy in discriminating T1 from T2 lesions. The combined results of large recent studies show a diagnostic accuracy for T3 and T4 tumours of 88–100%, with 64–85% accuracy for T2 lesions and 75–82% accuracy for T1 tumours.9,10 T1 lesions are often overstaged, probably because of submucosal fibrosis, connective tissue hyperplasia or ulceration. Diagnostic accuracy can be improved using mini-probes; however, complete assessment of larger lesions is difficult and bears the risk of underestimating the deepest invasion when parts of the lesion are missed and subsequently not assessed.11 Many endoscopists rely, for these reasons, on endoscopic assessment alone followed by histopathological assessment of the resected specimen. A German study demonstrated a similar diagnostic accuracy of 83.4% and 79.6% using either high-resolution endoscopy or endosonography, respectively, in assessing infiltration depth in early neoplasia in the oesophagus.12 The authors believe the same applies for early neoplasia in the stomach. Large discrepancies between Western and Japanese pathologists in the diagnostic criteria for adenoma, dysplasia and carcinoma in the gastrointestinal tract have led to considerable problems in the comparison between Western and Japanese data. This led to a consensus meeting in 1998 in Vienna, ultimately resulting in the Vienna classification of gastrointestinal epithelial neoplasia and the revised Vienna classification in 2002.13 This classification allows not only for a more universal nomenclature of gastrointestinal epithelial neoplasia but also corresponds more properly with clinical management. The revised Vienna classification is shown in Table 8.3. Table 8.3 The revised Vienna classification of gastrointestinal epithelial neoplasia The basic principle of what is nowadays termed endoscopic mucosal resection (EMR) is the removal of a mucosal lesion by resecting it from its deeper layers using a snare instrument. This method does not allow for lesions larger than 2 cm to be removed en bloc.14 Larger lesions can be removed by EMR, but only in a piecemeal fashion. The technique is fundamentally different from ESD, where the submucosal layer is carefully and stepwise dissected. Using EMR, early neoplasia is often lifted from the proper muscle layer before resection using different solutions of saline for submucosal injection.15–17 The lesions can then be sucked into a cap that is placed at the tip of the endoscope with a snare preloaded on to the rim of the cap. After sucking the lesion into the cap, the snare is pulled. The content of the snare is then resected using high-frequency current. An alternative resection method that is frequently used is the so-called ‘band-and-cut’ method. A lesion is sucked into a modified multiband ligator. By ligating the mucosa bearing the lesion, a pseudopolyp is created that can be resected using a snare. This multiple band mucosectomy allows for larger segments of neoplasia to be completely resected.18 This method is increasingly used in larger Barrett’s segments but is also applicable in the cardia and the antrum of the stomach. Endoscopic submucosal dissection (ESD) was originally developed in Japan for the local treatment of superficial EGC limited to the mucosal layer or with a minimal invasion of the submucosal layer. The main goal of submucosal dissection is to retrieve the lesion en bloc for histopathological staging and to minimise the chance for local recurrence. ESD is performed in several steps. First, the lesion is delineated by placing circumferential dots using electrocautery around the lesion with a few millimetres of free margin. The lesion is then lifted from the proper muscle layer by submucosal injection in the same fashion as in EMR.15–17 The solutions used are often stained with either indigo carmine or methylene blue. This dye will colour the submucosal layer and facilitate recognition of the separate layers and blood vessels. After lifting the lesion a circumferential incision of the mucosal layer is made, placing the markers just inside the circumferential cut. From this point onwards the submucosal layer is carefully dissected from the muscle layer, often with additional submucosal injections. In Fig. 8.2 an example of a Paris 0-IIa + IIc is shown, which is subsequently removed by ESD. Different speciality knives have been developed for ESD. A breakthrough in ESD was the development of the insulated tip (IT) knife in 1996.14 This is a speciality endoscopic knife with an insulated tip. An insulated small ceramic sphere is mounted on the top of a high-frequency needle knife, allowing safe and easy incision and separation of the mucosal and submucosal layers. Subsequently, the design of the original IT knife has been further adapted leading, for example, to the IT-2 knife, the hook knife, the flex knife, the triangular tip (TT) knife and the hybrid knife, the latter combining radiofrequency with a water-jet application.19 This novel technique combines radiofrequency with a distance-dependent water-jet application, allowing for easier and safer submucosal lifting and cutting in ESD.20,21 Figure 8.2 Early gastric cancer removed by ESD. (a) A Paris 0-IIc + IIa early gastric cancer located in the antrum. (b–e) Markings are placed around the lesion (b) and after submucosal lifting and circumferential incision (c), the lesion is stepwise dissected (d) and finally fully removed (e). Histopathological assessment demonstrated a well-differentiated adenocarcinoma confined to the mucosal layer with a maximum extent into the muscularis mucosae and tumour-free resection margins. The aim of both EMR and ESD is to strive for complete resection of the neoplastic lesion. Routinely, after endoscopic resection, the resected specimens are stretched and pinned on cork or paraffin and sent for pathological assessment. This final staging step by the pathologist should provide the necessary information on (i) quantitative criteria (lateral margins, deeper margins, maximum extent of submucosal invasion) and (ii) qualitative criteria (differentiation grade, lymphovascular involvement), which correspond with the risk of lymph node metastasis.7,8 This ultimate information is pivotal for further management. It mandates additional surgery or allows for a surveillance policy. Because of an approximately 14% chance of metachronous gastric cancer over a 5-year period23 and possible remnant neoplastic tissue, surveillance is usually carried out after endoscopic resection at 6-month intervals during the first year, followed by annual surveillance thereafter. Early detection of metachronous neoplasia can be treated by repeated endoscopic resection. Most bleeding complications occur during the procedures and can be dealt with instantly. Superficial bleeding vessels are identified and treated either by using coagulation, adrenaline injections or clips. Delayed bleeding also occurs and might necessitate subsequent re-intervention. A large prospective study found no risk factors for bleeding besides the presence of a gastric malignancy itself.24 In expert hands, perforations occur during EMR and ESD in about 0.2% and 1–4% of cases, respectively. A recent European study reported a 20% perforation rate after ESD at a tertiary referral centre.25 This study clearly demonstrates a difference in expertise in some Western referral centres compared to Japanese and Korean expert centres. This is mostly due to a much lower incidence of EGCs in the Western world, resulting in an insufficient exposure to this type of pathology and resection techniques. Recently, a panel of European experts has attempted to set standards for quality criteria for ESD in European countries.26 Perforations can lead to pneumoperitoneum and in severe cases to generalised peritonitis. Usually, perforations are recognised immediately during the procedure and endoscopic management is possible and frequently adequate.27 Closure with clips is a safe treatment option together with nasogastric drainage and fasting.28 Recently, in a small case series the use of the over-the-scope clip (OTSC) was described for closure of perforations in a clinical setting.29 The OTSC device is a promising novel technique with a reported closure success rate of 92%.
Endoscopic and surgical treatment of early gastric cancer
Introduction
Risk and development of early gastric cancer
Classification of early gastric cancer
Endoscopic appearance
Lymph node metastasis
Endosonography
Revised Vienna classification
Category
Diagnosis
Clinical management
1
Negative for neoplasia
Optional follow-up
2
Indefinite for neoplasia
Endoscopic follow-up
3
Mucosal low-grade neoplasia
Endoscopic resection or follow-up
Low-grade dysplasia
Low-grade adenoma
4
Mucosal high grade neoplasia
Endoscopic or local surgical resection
4.1
High-grade adenoma/dysplasia
4.2
Non-invasive carcinoma (carcinoma in situ)
4.3
Suspicious for invasive carcinoma
4.4
Intramucosal carcinoma
5
Submucosal invasion by carcinoma
Surgical resection
Endoscopic treatment
Endoscopic submucosal dissection
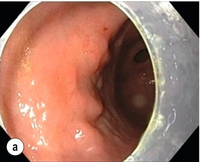
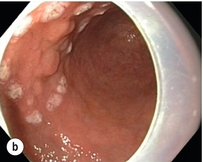
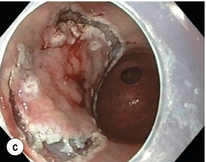
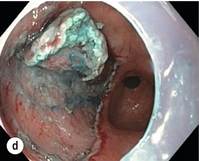
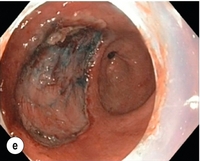
Complete resections
Complications of endoscopic resections
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endoscopic and surgical treatment of early gastric cancer