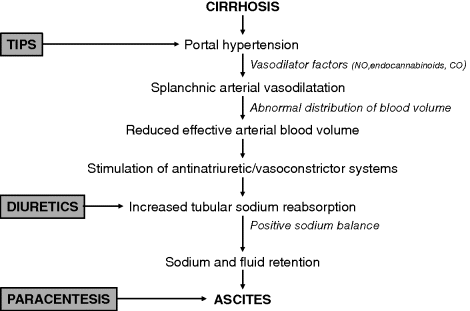
Chapter 14 Andrés Cárdenas,1 Isabel Graupera,2 and Pere Ginès2 1GI Unit, Hospital Clínic and University of Barcelona, Institut d’Investigacions Biomèdiques August Pi-Sunyer (IDIBAPS), Ciber de Enfermedades Hepaticas y Digestivas (CIBERHED) 2Liver Unit, Hospital Clínic and University of Barcelona, Institut d’Investigacions Biomèdiques August Pi-Sunyer (IDIBAPS), Ciber de Enfermedades Hepaticas y Digestivas (CIBERHED), Instituto Reina Sofía de Investigación Nefrológica (IRSIN) Barcelona Spain Cirrhosis is the twelfth leading cause of mortality in the United States, accounting for nearly 32 000 deaths in 2010 [1]. Ascites is the most common complication of cirrhosis resulting in poor quality of life, high risk of development of other complications, increased morbidity and mortality associated with surgical interventions, and poor long-term outcome [2–6]. Cirrhotic patients with first onset ascites have survival rates of 85% at 1 year and 56% at 5 years without liver transplantation [3]. The practical management of ascites involves a proper evaluation of the patient with a thorough history and physical examination. In addition, complete laboratory, ascitic fluid, radiologic, endoscopic, and, in some cases, histologic tests should be performed. One of the most important steps in the initial assessment of patients with ascites is referral of appropriate candidates for liver transplantation as this offers a definitive cure of cirrhosis and its complications. Taking proper care of patients with ascites can be challenging because they are prone to complications such as spontaneous bacterial peritonitis (SBP), hypervolemic hyponatremia, hepatic hydrothorax, hepatorenal syndrome (HRS) and hepatocellular carcinoma, bleeding from esophageal or gastric varices, and hepatic encephalopathy. While the initial management of uncomplicated ascites with low sodium diet and diuretic treatment is straightforward in the majority of patients, there is a group of patients that fails to respond to diuretics and become a real therapeutic challenge. This chapter focuses on the practical aspects of the evaluation and treatment of patients with ascites and cirrhosis. The major factor contributing to ascites formation is splanchnic vasodilatation resulting in a decreased effective arterial blood volume [7]. Increased hepatic resistance to portal flow due to cirrhosis causes a progressive increase in portal pressure with collateral vein formation and shunting of blood to the systemic circulation. As portal hypertension develops, splanchnic arterial vasodilatation occurs due to the enhanced local production of nitric oxide and other vasodilators including carbon monoxide and endogenous cannabinoids as a consequence of endothelial stretching and bacterial translocation [8–10]. Evidence indicates that bacterial translocation to mesenteric lymph nodes with increased production of bacterial products and subsequent stimulation of cytokine synthesis has a major role in the pathogenesis of arterial vasodilatation and the associated circulatory abnormalities that occur in cirrhosis [10,11]. In the early stages of cirrhosis, splanchnic arterial vasodilation is moderate and has no major effect on effective arterial blood volume, which is maintained within normal limits due to an increase in plasma volume and cardiac output [7]. In advanced stages, splanchnic arterial vasodilatation is so intense that effective arterial blood volume becomes markedly reduced and arterial pressure falls. A reduction in cardiac output occurs in the late stages of cirrhosis and also contributes to the impairment in effective arterial blood volume [12–14]. As a consequence of the impairment in effective arterial blood volume, there is homeostatic activation of vasoconstrictor and antinatriuretic factors to maintain arterial pressure, resulting in renal sodium and fluid retention. The combination of portal hypertension and splanchnic arterial vasodilation alters intestinal capillary pressure and permeability, which facilitates the accumulation of the retained fluid in the abdominal cavity. With disease progression, there is a marked impairment in renal solute-free water excretion and renal vasoconstriction, leading to hypervolemic hyponatremia and hepatorenal syndrome, respectively. The pathophysiologic rationale for the treatment of patients with cirrhosis and ascites is described in Figure 14.1. Figure 14.1 Pathogenesis of ascites formation in cirrhosis and current therapeutic options for ascites and dilutional hyponatremia. Portal hypertension is the main factor responsible for the development of splanchnic vasodilation and a decrease in effective arterial blood volume (NO, nitric oxide; CO, carbon moxide). The transjugular intrahepatic portosystemic shunt (TIPS) by reducing portal pressure is an effective treatment of ascites, particularly in patients with refractory ascites. The compensatory homeostatic response occurs secondary to a reduced effective arterial blood volume and leads to the activation of antinatriuretic factors (mainly the angiotensin–aldosterone system) with subsequent sodium retention. The use of diuretics (spironolactone) that promote natriuresis are the preferred therapy for patients with sodium retention and fluid accumulation. Patients with large-volume ascites should be treated with therapeutic paracentesis and albumin administration followed by diuretics as tolerated. Patients with cirrhosis presenting with ascites must always be questioned about precipitating events such as excessive salt intake, alcohol consumption, infections, medications such as nonsteroidal anti-inflammatory drugs or steroids, and noncompliance with diuretic therapy if they previously had ascites. Worsening liver disease, portal vein thrombosis, parenchymal renal failure (i.e., glomerular diseases), and development of hepatocellular carcinoma (most often associated with tumor invasion of the portal vein) may also precipitate the development of ascites. More commonly, ascites develops insidiously over the course of weeks. The main symptoms are an increase in abdominal girth often accompanied by lower extremity edema [15]. In patients with a large amount of ascites, respiratory function and physical activity may be impaired. Dyspnea may occur as a consequence of increasing abdominal distention and/or accompanying pleural effusions. Patients with SBP can present with fever, chills, abdominal pain, encephalopathy, renal failure, and rebound abdominal tenderness. However, patients with SBP may be asymptomatic and the diagnosis relies on the examination of peritoneal fluid. Other common manifestations of patients with ascites include colicky abdominal pain, anorexia, malaise, weakness, malnutrition, and/or jaundice. Increased intrabdominal pressure favors the formation of abdominal hernias in patients with cirrhosis and long-standing ascites [15]. Umbilical hernias increase in size if ascites is not treated and sometimes cause significant complications such as strangulation and rupture due to previous ulcer formation on the surface. Rupture may be complicated with infection of the ascitic fluid and delayed wound healing. Inguinal hernias can also be problematic in patients with ascites. The best treatment is prevention of ascites because surgical treatment is usually not recommended in those with advanced cirrhosis. Muscle cramps are a common clinical feature in patients with cirrhosis and ascites. The prevalence of muscle cramps (>3 episodes per week) in cirrhotic patients is approximately 50–60% and is related to the duration of cirrhosis and severity of impairment in circulatory function and presence of ascites. Muscle cramps commonly occur in the lower extremities and can significantly impair the patients’ quality of life. Painful gynecomastia may occur in patients with cirrhosis and ascites. The exact mechanisms are unknown; however, it is postulated that estrogen excess in cirrhotic patients and the estrogenic effects of the spironolactone are possible causes [15]. Most patients are malnourished and have signs of muscle wasting and atrophy. Other common findings are palmar erythema, spider nevi, jaundice, loss of body hair, white nails (Terry nails), splenomegaly, inguinal and umbilical hernias, and lower extremity edema [15]. The physical examination is not completely reliable for detecting fluid in abdominal cavity. Patients must have approximately 1500 mL of fluid to be detected reliably by examination [16]. The abdomen is distended with bulging flanks; dullness to percussion in these areas indicates ascites. When free fluid is present in the abdominal cavity, it moves to the flanks and the intestines float upward when the patient is supine. The air–fluid level is higher than that normally found on the lateral aspect of the abdomen, if the patient is turned on his/her side the dullness will shift and percussion over the uppermost part becomes tympanic, because that area is occupied by intestines as fluid shifts to the other side. This maneuver is called shifting dullness and is very sensitive for detecting ascites [16]. Mild or moderate pleural effusions are also common in patients with ascites. Large pleural effusions, usually greater than 500 mL, in cirrhotic patients without cardiopulmonary disease are uncommon and are known as hepatic hydrothorax. The current quantitative classification of ascites divides patients into three groups. In grade 1 ascites, fluid is detected only by ultrasound, in grade 2, ascites is moderate with symmetrical distention of the abdomen, and in grade 3, ascites is large or tense with marked abdominal distention [17,18]. The evaluation of patients with ascites should include standard hematology, electrolyte, renal (serum creatinine and blood urea nitrogen, and urinary sodium), coagulation (prothrombin time, PT, or international normalized ratio, INR), and liver tests (aminotransferases, bilirubin, albumin, total protein, alkaline phosphatase, gamma-glutamyl transferase) [17–19]. An abdominal ultrasonography to rule out hepatocellular carcinoma and evaluate the patency of the portal venous system should be performed in all patients. In addition, an upper gastrointestinal endoscopy to assess the presence and characteristics of esophageal and gastric varices is recommended. In patients with renal failure (serum creatinine >1.5 mg/dL), urine sediment, and 24-hour urine protein should be assessed and the kidneys examined by ultrasonography. Evaluation of circulatory function should include measurement of arterial pressure and heart rate. A diagnostic paracentesis (30 mL of fluid) is required in all patients presenting with their first episode of ascites, and all patients with any evidence of clinical deterioration such as fever, abdominal pain, gastrointestinal bleeding, hepatic encephalopathy, hypotension, or renal failure. The ascitic fluid in cirrhotics is mostly transparent and yellow–amber in color. Tests on the ascitic fluid should include cell count, albumin, total protein and cultures in blood culture bottles (10 mL of fluid injected at the bedside) [17–20]. The cell count is the most helpful test in determining bacterial infection. In patients without infection of the ascitic fluid, the ascitic fluid white blood cell count is <100/mm3 with a predominance of mononuclear cells (>75%) and a low number of neutrophils. An increased number of white blood cells with predominance of neutrophils indicates peritoneal infection [18,21]. The diagnosis of SBP is made when the fluid sample has >250/mm3 neutrophils and there are no signs of peritonitis due to perforation or inflammation of intra-abdominal organs. The difference between serum albumin concentration and ascites albumin concentration (serum–ascites albumin gradient) in patients with cirrhosis and ascites is usually >11 g/L, values <11 g/L suggest a cause of ascites other than cirrhosis [22]. A low total protein concentration in ascitic fluid (<15 g/L) is associated with an increased risk of SBP and in selected patients may indicate a need for antibiotic prophylaxis with oral quinolones to reduce the risk of SBP and HRS. Finally, cultures of ascitic fluid of patients with ascitic fluid infection in blood culture bottles increase the probability of isolating an organism to approximately 50% of cases if the fluid is inoculated into the bottles at the bedside [21,23]. A low sodium diet of approximately 80–120 mmol/day (i.e., no added salt and no preprocessed meals) facilitates the elimination of ascites and delays the reaccumulation of fluid [18]. However, a reduction in sodium intake alone only achieves a negative sodium balance and complete elimination of ascites in only 10% of patients unless diuretics are given. Fluid restriction is not necessary unless patients have hypervolemic hyponatremia (defined as serum sodium <130 mEq/L together with ascites and/or edema). An evaluation by a nutritionist is of utmost importance for appropriate education regarding an appropriate caloric and salt intake. Improvement of the nutritional status is essential because patients with advanced liver disease have decreased intake and absorption of nutrients, increased energy expenditure, and altered fuel metabolism with an accelerated starvation metabolism [24,25]. Nutritional therapy in cirrhotic patients can improve nutritional status, reduce infection rates, and decrease perioperative morbidity [24]. The goal is for nutritional supplementation to correct the underlying protein energy malnutrition. In advanced cases of malnutrition, supplemental enteral nutrition in cirrhotic patients with ascites may improve liver function and hepatic encephalopathy [24–27]. Patients with grade 1 ascites do not require any specific treatment but they should be cautioned about avoiding foods with large amounts of salt. Patients with grade 2 ascites are usually treated with sodium restriction and diuretics. Patients with grade 3 ascites are managed with large-volume paracentesis along with diuretics. Patients with refractory ascites do not respond to high doses of diuretics or develop side effects that preclude their use. The discussion of the management of ascites may therefore be divided in three parts: moderate-volume ascites (grade 2 ascites), large-volume ascites (grade 3), and refractory ascites. A summary of recommendations for the management of ascites is included in Table 14.1. Table 14.1 Management practice points in patients with ascites and cirrhosis. Start with a low-sodium diet (90 mmol/day) and spironolactone (50–100 mg/day) to reach goal of weight loss: 300–500 mg/day. If needed, doses to be increased every 7 days up to 400 mg/day spironolactone. Furosemide can be added at a starting dose of 20–40 mg/day and subsequently increased to 160 mg/day if needed Total paracentesis plus intravenous albumin (8 g/L of ascites removed) followed by a low-sodium diet (90 mmol/day) and diuretics if patient tolerated them beforehand Total paracentesis plus intravenous albumin can be performed as needed. Consider use of TIPS in patients with very frequent recurrent ascites, very rapid recurrence of ascites, and preserved liver function (bilirubin <3 mg/dL, platelet count >75 000, serum sodium level >130 mEq/L, Child–Pugh score <13, model for end-stage liver disease (MELD) score <18), aged <70 years, without hepatic encephalopathy, central or large hepatocellular carcinoma or cardiopulmonary disease Patients with moderate ascites usually accumulate fluid slowly, such that they typically do not develop large-volume ascites unless their sodium intake is very high or they do not seek medical attention for long periods of time. Renal solute-free water excretion and glomerular filtration rates are normal in most cases; therefore, serum sodium and creatinine concentration are usually within normal limits. These patients typically can be managed as outpatients and do not require admission unless they have other complications of cirrhosis. A negative sodium balance with loss of ascites is obtained in most cases with low doses of diuretics. Diuretics eliminate the excess extracellular fluid presenting as ascites and edema by increasing renal sodium excretion together with fluid excretion. The diuretics most frequently used in patients with cirrhosis and ascites are aldosterone antagonists, mainly spironolactone and potassium canrenoate, drugs that selectively antagonize the sodium-retaining effects of aldosterone in the renal collecting tubules, and loop diuretics, especially furosemide, that inhibit the Na+-K+–2Cl− cotransporter in the loop of Henle. Diuretic therapy is effective in the elimination of ascites in 80–90% of patients with ascites, a percentage that increases up to 95% if only patients without renal failure are considered [18]. Diuretics are indicated for all patients with grade 2 ascites. Patients with their first episode of ascites usually respond with spironolactone 50–100 mg/day [18,19,28–30]. Patients with recurrent episodes of ascites should receive the combination of spironolactone 100 mg/day with furosemide 40 mg/day. If there is no response, compliance with diet and medications should be confirmed and diuretics may then be increased every 7 days by doubling doses (1 : 1 ratio) to a maximal dose of spironolactone (400 mg/day) and a maximal dose of furosemide (160 mg/day). The goal of treatment is to produce an average weight loss of 500 g/day in patients without peripheral edema and 800–1000 g/day in those with peripheral edema until ascites has decreased markedly and afterwards diuretic dosage can be reduced. This is in order to avoid overdiuresis and side effects. In patients who do not respond, compliance with low sodium diet and diuretics should always be assessed. In these cases, measurement of urine sodium, which provides an accurate assessment of the response to diuretics, may help in deciding whether there is a need to increase dosage.
Ascites
Pathophysiology of Ascites
Patients’ History
Physical Examination
Patient Evaluation
Management of Patients with Ascites
General Measures
Specific Measures
Moderate-Volume Ascites
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








