Key points
- •
Managing low-grade prostate cancers is a significant challenge, brought on by widespread adoption of prostate-specific antigen screening.
- •
Physicians who diagnose this disease and discuss treatment options with patients may move toward correctly titrating management to fit low-risk patients by shifting the classification of low-risk prostate cancers into another diagnostic category, such as a benign tumor or a tumor of low malignant potential.
- •
This shift is problematic in a biological sense, because low-grade prostate cancers clearly fulfill well-established definitions of cancer, and it would also be problematic in a practical sense, because risk classification by Gleason grading is often inaccurate and low-grade cancers may progress to high-grade cancers.
- •
In the proposed system, indolent cancers currently scored as Gleason 6 out of 10 would be assigned a score of 1 out of 5.
- •
The more accurate risk assessment provided by the new scoring system would be the start of a discussion between patients and physicians on how best to manage a cancer that is likely harmless.
The science of diagnosing cancer
The Hallmarks of Cancer
The idea that cancer can be indolent leads to the question, what is cancer? A review published in 2000 entitled The Hallmarks of Cancer , and its more recent revision, focus on a variety of properties of cancer, including the ability to grow autonomously, evade signals that would cause programmed cell death in normal cells, invade, and metastasize ( Tables 1 and 2 ). The hallmarks provide a useful heuristic device for discussing cancer biology, particularly in the context of basic research. However, hallmarks are not strict criteria for diagnosing cancer. In fact, as shown in Table 1 , many of the hallmarks are features of benign neoplasms and therefore cannot be used to define cancer. As carefully delineated in medical textbooks and entertainingly presented by Lazebnik, cancer is a malignant neoplasm. A neoplasm (benign or malignant) is an autonomously growing clone of cells; only malignant neoplasms invade or metastasize. In practice, invasion is the only feature that is both necessary and sufficient for the diagnosis of cancer (see later discussion). Aside from invasion and metastasis, the other hallmarks are found in benign neoplasms and, therefore, not defining features of malignancy (see Table 1 ).
| Proliferative Signals | Evading Growth Suppressors | Immortality | Angiogenesis | Invasion | Metastasis | |
|---|---|---|---|---|---|---|
| Normal growth and homeostasis | + | − | − | + | − | − |
| Benign neoplasms | + | + | + | + | − | − |
| Cancers | + | + | + | + | + | +/− |
| Category | Feature | Benign | PIN | Low-Grade Prostate Cancer | Reference |
|---|---|---|---|---|---|
| Architecture | Glandular profiles | Large, folded, evenly spaced | Large, folded, evenly spaced | Small, round, simple | |
| Basal cell layer | Present | Present, but attenuated | Absent | ||
| Gene expression | Intense AMACR immunoreactivity on biopsy | <21% | 42%–56% | >90% | |
| Chromosomal alteration | D8S87 loss on chromosome 8p12 | Infrequent | Infrequent | 20%–50% | |
| TMPRSS2-ERG fusion | 0% | 12% | 39%–50% | ||
| PTEN alteration (any) | 5% | 15% | 31% |
Metastasis Is Not a Reliable Defining Feature of Cancer
Although almost all metastasizing tumors are malignant, there are malignant tumors (cancers) that almost never metastasize. These nonmetastasizing cancers can be highly locally invasive and lethal. For example, high-grade glioma brain tumors rarely metastasize, but are rapidly and almost uniformly lethal. On the other side of the aggressiveness spectrum, there are several invasive cancers that carry an exceedingly low risk of metastasis. The most salient and common example is basal cell carcinoma (BCC) of the skin. Arising in sun-exposed areas, BCC is almost never fatal and carries a minuscule risk of metastasis, estimated to be as low as 0.0028% of cases. However, it can be highly invasive locally, causing disfigurement and loss of function, especially around the eyes and scalp. Therefore cancers vary in their potential to metastasize, but are defined primarily by their ability to invade.
Invasive Features Distinguish Prostatic Intraepithelial Neoplasia from Prostate Cancer
Prostate cancer commonly develops from a preinvasive precursor lesion called prostatic intraepithelial neoplasia (PIN). PIN consists of neoplastic epithelial cells within preexisting benign glands. For epithelial cancers such as prostate cancer, invading through the basement membrane defines cancer, and low-grade (ie, Gleason ≤6) prostate cancers clearly fit this criterion. The architectural features of PIN glands are those of benign prostate acini. By contrast, cancers of Gleason score 6 have an infiltrative pattern extending irregularly into the stroma, not only between benign glands but also beyond the prostate into extraprostatic tissue ( Fig. 1 A ). In contrast to carcinoma, neither PIN nor benign prostate glands ever infiltrate beyond the prostate.
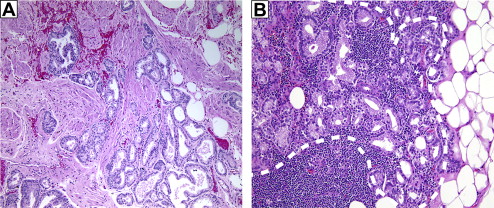
The replicative process of benign prostate epithelia is quite different from that of cancer. In benign prostate, basal cells give rise to luminal secretory cells as part of a differentiation program. Prostate cancer cells, by contrast, replicate autonomously. The distinctive architectural features of low-grade prostate cancer evolve as neoplastic cells invade the basement membrane surrounding PIN glands to form new glands in the surrounding stroma. The newly formed cancer glands lack a surrounding basal cell layer. By contrast, although not always easy to identify in diagnostic specimens, both benign glands and PIN glands are surrounded by a layer of basal cells adjacent to the basement membrane.
Molecular Features Distinguish Prostatic Intraepithelial Neoplasia from Prostate Cancer
The process by which noninvasive neoplastic cells acquire invasive potential is unclear. Accordingly, there are no molecular tests that accurately distinguish an invasive cancer cell from a noninvasive, in situ precursor. Nevertheless, PIN and low-grade cancers have distinctive molecular features (see Table 1 ). In addition to lacking basal cells and having smaller, simpler glandular profiles than PIN, low-grade prostate cancers express higher levels of α-methylacyl-CoA racemase (AMACR). Higher AMACR expression seems to facilitate lipid oxidation, a favored energy source for prostate cancer cells, suggesting qualitatively or quantitatively different energetic requirements for invasive growth. At the chromosomal level PIN and cancer share a variety of alterations, but PIN seems less likely to lose the microsatellite repeat D8S87 on proximal 8p, or to show intrachromosomal rearrangements (fusions) between the TMPRSS2 and ERG genes. The biological significance of TMPRSS2-ERG fusions is currently unclear, but they are emerging as potentially useful diagnostic features.
By contrast, the tumor suppressor PTEN has a well-established role in the biology of prostate cancer. Genomic alterations such as gain, monosomy, and hemizygous or homozygous loss at the PTEN locus are detected twice as often in Gleason-6 prostate cancer (31%) than in PIN (15%). PTEN restrains cancer growth and invasion by its inhibitory effects on downstream genes in the PI3 kinase and AKT signaling pathways. From research in animal models and clinical samples, complete inactivation of PTEN is a critical step in the formation and metastasis of prostate cancer. In humans PTEN loss is an established event in advanced cancers, whereas other kinds of PTEN alterations, including partial loss, have been recently recognized in PIN and in early prostate cancer, although their significance is unknown. To better delineate the molecular basis of human prostate cancer, additional studies will need to interrogate the relationship between partial PTEN loss and activity of the downstream pathways that PTEN is responsible for restraining. In contrasting PIN and early prostate cancer, these studies may reveal more specific ways to distinguish between the two entities. In summary, although no single molecular feature perfectly distinguishes a PIN cell from a cancer cell, there are striking architectural and biological differences, and a variety of molecular features (summarized in Table 2 ) that are significantly more pronounced in cancer.
Intraductal Carcinoma of the Prostate
Special consideration is merited for a relatively rare entity, called intraductal carcinoma of the prostate (IDC-P) (reviewed by Robinson and Epstein ). Although not always evident on biopsy, affected cases almost always have an invasive component in addition to the IDC-P. For this reason, IDC-P is believed in most cases to represent cancerization of benign prostatic ducts by high-grade prostate cancer. Thus, IDC-P cells can reasonably be labeled cancer cells, even though they are a by-product of invasive cancer and are not invasive themselves. In the uncommon cases (∼10%) where IDC-P is not associated with infiltrating carcinoma, it represents a precursor lesion with a high likelihood of developing high-grade invasive cancer if left untreated, such that it still warrants the term IDC-P.
The Race to the Basement
Two recent reviews have argued that Gleason-6 cancers are not cancer. This opinion was based on observations that Gleason-6 cancers are not only indolent but also lack certain molecular features of higher-grade cancers that can be aligned with the hallmarks of cancer and, perhaps most importantly, the claim that these cancers do not invade surrounding tissues. Although the discussion above has dispensed with the idea that other cancer hallmarks can be used to define cancer (see earlier discussion and Table 1 ), the idea that low-grade cancers do not invade deserves extra scrutiny. Supporting evidence for this idea included the fact that low-grade prostate cancers rarely grow when transplanted into mice, and that there is less development of basement membrane around high-grade cancers than there is around low-grade cancers. However, the ability to grow in mice is not a useful defining feature of cancer, as even high-grade and metastatic prostate cancers also often fail to grow as transplants. It is also well established that invasive prostate cancer cells, both primary and metastatic, produce new basement membrane. Therefore, the presence or absence of basement membrane around a cell is not a reliable indicator of invasiveness or malignancy. Of equal or greater importance, Gleason-6 cancers can be observed in extraprostatic tissue (see Fig. 1 A). This observation indicates that these cancer cells not only escaped their original basement membrane, but invaded through benign tissue to escape the prostate. It would seem that, this evidence fulfills any logical definition of invasiveness as applied to cancer.
Which Prostate Cancer Cells Invade and Metastasize?
Lavery and Droller present a somewhat more radical argument that Gleason-6 cancers do not evolve into higher-grade cancers. Instead, PIN can evolve into either a pattern-3 clone or a pattern-4 clone. In their model, pattern-3 clones give rise to a noninvasive lesion, akin to PIN. Only pattern-4 clones are invasive in this model, and they arise through a distinct morphologic and molecular pathway. As supporting evidence, the investigators cited studies of men with metastases after prostatectomy in which multiple cancers were found in the prostate, but only a single clone from each cancer gave rise to metastases. The investigators illustrate an interesting question regarding prostate cancer clones that give rise to metastases: If Gleason 3 + 3 = 6 cancers cannot metastasize, then how do Gleason 3 + 4 = 7 cancers metastasize?
The two alternatives are that Gleason 3 + 3 = 6 cancers can evolve into Gleason 3 + 4 = 7 cancers, or that Gleason pattern 4 evolves independently. Both pathways are possible. A recent genomic study found that patterns 3 and 4 in Gleason 3 + 4 = 7 cancers are essentially clonal. This conclusion agrees with common observations in prostate pathology. In examining prostate cancer metastases, one can find pattern-3 glands admixed with higher-grade glands (see Fig. 1 B). Because only cancers with pattern 4 or 5 can metastasize, this observation indicates that a single cancer cell can produce both low-grade and higher-grade progeny. Thus low-grade and high-grade glands are likely two differentiation states in the same pathway. Furthermore, Gleason grade is highly correlated with tumor volume ; the most parsimonious model would be one in which low-grade tumors evolve into high-grade tumors as they grow. However, one could also explain the data based on the increased growth rate of higher-grade carcinomas. It has also been demonstrated that high-grade carcinoma can arise de novo without going through a pathway of dedifferentiation of lower-grade cancers. There are patients who have been followed for many years with cancer of Gleason score 6, who then are found to have higher-grade cancer. The unresolved issue is whether these high-grade cancers represent progression of the previously diagnosed lower-grade cancer or emergence a new tumor. It can be argued that if Gleason score 3 + 4 = 7 or 4 + 3 = 7 is cancer whereby a prognostic component of the cancer is the pattern 3, then when a tumor is pure pattern 3 (ie, Gleason score 3 + 3 = 6) why would it not still be cancer?
The science of diagnosing cancer
The Hallmarks of Cancer
The idea that cancer can be indolent leads to the question, what is cancer? A review published in 2000 entitled The Hallmarks of Cancer , and its more recent revision, focus on a variety of properties of cancer, including the ability to grow autonomously, evade signals that would cause programmed cell death in normal cells, invade, and metastasize ( Tables 1 and 2 ). The hallmarks provide a useful heuristic device for discussing cancer biology, particularly in the context of basic research. However, hallmarks are not strict criteria for diagnosing cancer. In fact, as shown in Table 1 , many of the hallmarks are features of benign neoplasms and therefore cannot be used to define cancer. As carefully delineated in medical textbooks and entertainingly presented by Lazebnik, cancer is a malignant neoplasm. A neoplasm (benign or malignant) is an autonomously growing clone of cells; only malignant neoplasms invade or metastasize. In practice, invasion is the only feature that is both necessary and sufficient for the diagnosis of cancer (see later discussion). Aside from invasion and metastasis, the other hallmarks are found in benign neoplasms and, therefore, not defining features of malignancy (see Table 1 ).
| Proliferative Signals | Evading Growth Suppressors | Immortality | Angiogenesis | Invasion | Metastasis | |
|---|---|---|---|---|---|---|
| Normal growth and homeostasis | + | − | − | + | − | − |
| Benign neoplasms | + | + | + | + | − | − |
| Cancers | + | + | + | + | + | +/− |
| Category | Feature | Benign | PIN | Low-Grade Prostate Cancer | Reference |
|---|---|---|---|---|---|
| Architecture | Glandular profiles | Large, folded, evenly spaced | Large, folded, evenly spaced | Small, round, simple | |
| Basal cell layer | Present | Present, but attenuated | Absent | ||
| Gene expression | Intense AMACR immunoreactivity on biopsy | <21% | 42%–56% | >90% | |
| Chromosomal alteration | D8S87 loss on chromosome 8p12 | Infrequent | Infrequent | 20%–50% | |
| TMPRSS2-ERG fusion | 0% | 12% | 39%–50% | ||
| PTEN alteration (any) | 5% | 15% | 31% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




