Hepatitis can be defined as the constellation of symptoms and signs resulting from hepatic inflammation and hepatic cell necrosis. If the insult is acute and occurs in a previously asymptomatic individual, the term acute hepatitis can be applied. The most common causes of acute hepatitis are viruses, toxins, and alcohol. Occasionally other disease entities such as Wilson’s disease, leukemias, and lymphomas with acute infiltration of the liver may give rise to a clinical picture of acute hepatitis. Viruses, however, are the major etiologic agents of acute liver injury.
Systemic infection with several viruses results in hepatic inflammation and cell death. Viruses that cause hepatitis have been classified as hepatitis A (HAV), B (HBV), C (HCV), delta (HDV), and E (HEV). However, in some individuals, infection with the Epstein-Barr virus (EBV) or cytomegalovirus (CMV) also results in acute hepatitis.
In most patients, acute viral hepatitis presents as an acute illness characterized by the abrupt onset of malaise, fever, anorexia, nausea, headache, abdominal discomfort, and pain. Jaundice, itching, dark-colored urine, and light-colored stools often cause the patient to seek medical attention. At this stage, the disease caused by different viruses is usually indistinguishable; serologic studies and viral DNA or RNA determination by polymerase chain reaction (PCL) may provide the only means of identification. Pertinent factors regarding the five forms of acute and chronic viral hepatitis are summarized in
Tables 50-1 and
50-2.
I. HEPATITIS ASSOCIATED WITH EPSTEIN-BARR VIRUS
A. Pathogenesis.
EBV is a herpes virus and is the causative agent of infectious mononucleosis (IM). Although clinical jaundice occurs in approximately 5% of the cases of IM, hepatitis caused by this virus may be as common as that from HAV and HBV. However, the hepatitis in IM is usually mild and is accompanied by other clinical features of IM.
1.
In most individuals, the natural primary infection with EBV occurs asymptomatically in childhood, conferring lifelong immunity to IM in the form of IgG antibodies to EBV. In the industrialized countries, 50% to 60% of the children by age 5 and more than 80% of the individuals by age 20 acquire seropositivity to EBV. Infection tends to occur earlier in lower socioeconomic groups, but symptomatic disease is frequent in college students who were not exposed to EBV in childhood. In adults, symptomatic disease develops in one half of the cases and may be severe in older individuals.
2. Transmission of the virus
occurs through contact with infected saliva. The virus is found in saliva droplets and in the epithelial cells. Blood transfusions have been implicated in a few cases.
3.
In most cases, the virus initially infects oropharyngeal epithelial cells with secondary infection of the B lymphocytes with further dissemination of the virus. The incubation period is usually 4 to 7 weeks. The virus can be isolated from oral secretions in 80% of patients with acute IM. The EBV genome is incorporated into some infected B lymphocytes, which incite a cytotoxic T-cell proliferation and response, seen in the peripheral blood smear as the atypical mononuclear cells of IM. Although the cellular and hormonal response brings the infection under control, infection persists in a subpopulation of B lymphocytes. In fact, EBV can be isolated from the saliva of 15% to 20% of asymptomatic adults. Persistent and reactivated infections are rare but do occur.
B. Clinical presentation.
In patients 15 to 30 years old, IM classically presents with fatigue, malaise, fever, anorexia, distaste for food and cigarettes, nausea, vomiting, and headache. Periorbital edema and a rash may be present. Sore throat is common and may be caused by a secondary infection with β-hemolytic Streptococcus. Lymphadenopathy and splenomegaly are present in about half of the cases. Ten percent to 15% of the patients have hepatomegaly, and 5% develop jaundice. Hemolytic anemia may be present.
Occasionally in IM, the hepatitis may be the predominant presentation, especially in older patients, and may last 1 to 2 months. In some cases, the clinical presentation of fever, right upper quadrant pain, jaundice, and elevated alkaline phosphatase may mimic extrahepatic obstruction.
1. Diagnostic studies.
Mild elevation of transaminases (more than twice normal) and alkaline phosphatase is common. Serum bilirubin is 1 to 8 mg/dL. The hepatitis is usually milder than seen with other viruses. Severe jaundice, liver failure, coagulopathy, and encephalopathy are rare.
In the acute disease, 60% of the patients have an absolute lymphocytosis, and 50% have the “atypical mononuclear cells.” The monospot test has a specificity of 99% and a sensitivity of 80%. It may be negative initially in one sixth of the patients and should be repeated. The heterophil antibody is more sensitive and is positive in 90% of the patients. EBV-specific serology (IgM antibody [Ab]) allows for definitive diagnosis but may be negative in early mild IM. A single high virus-specific antibody (IgG) does not reliably distinguish current from previous infection.
C. Treatment.
Currently there is no specific treatment for IM hepatitis. Corticosteroids may be of value in severe acute pharyngitis for the edema but do not provide any benefit for other manifestations of IM including hepatitis. Acyclovir sodium has been used in a few cases of persistent severe IM with some clinical improvement.
III. HEPATITIS A.
Infections with HAV account for approximately 25% of the clinical hepatitis cases diagnosed in industrialized nations. It occurs both sporadically and in epidemics. In general, it causes less morbidity and mortality than hepatitis virus types B, C, and delta.
A. Pathogenesis.
HAV is endemic in underdeveloped countries, where infections usually occur in children and are clinically inapparent. The outcome of the infection seems to depend on the age of the patient and the infecting dose of the virus. The disease is of shorter duration and milder in children. Adults may present with clinically significant disease; fulminant hepatitis occurs with a frequency of 1 to 8 per 1,000 cases. HAV is a 27-nm, nonenveloped RNA enterovirus and belongs to the group picornavirus. All strains of the virus identified to date belong to one serotype. The infection is acquired by the fecal-oral route and can be isolated from the liver, bile, stools, and blood during the late incubation period and acute preicteric phase of the disease. Fecal viral shedding and viremia diminish once jaundice occurs.
B. Diagnosis
1. Clinical presentation.
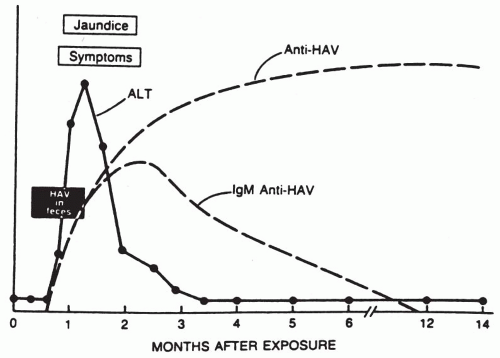
The typical course of a case of acute hepatitis A is shown in
Figure 50-1. Hepatitis A has an incubation period of from 2 to 7 weeks with a mean of 4 weeks. In clinically apparent cases, there is an abrupt onset of symptoms. Malaise, fever, anorexia with aversion to food and cigarettes, nausea, and abdominal pain are frequent. Within a few days after the onset of symptoms, there is a marked rise in serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin levels, resulting in jaundice. In approximately 25% of the cases, hepatosplenomegaly is noted.
2. The duration of the illness
is usually less than 1 month, but elevated serum transaminases have been recorded for as long as 6 months.
3. Complications from hepatitis A
are rare. A cholestatic phase may occur in some patients and may mimic obstructive jaundice. Relapses have been seen within 4 to 15 weeks of recovery from the disease and may be precipitated by exertion or alcohol intake. The disease may last 16 to 40 weeks. There may be complications from immune complex formation and deposition. There is no evidence that HAV causes a chronic carrier state or chronic liver disease; however, chronic carriers of HBV can be infected with HAV.
4. Serology.
The development of serologic assays for hepatitis A antigen (HAAg) and antibody (Haab) has allowed for accurate diagnosis of HAV infection. HAAg can only be detected in the stool and possibly the blood of viremic patients shedding the virus in the preclinical stages of the disease.
High titers of HAV-specific IgM antibody develop in all patients by the time of clinical presentation. Thus, the presence of anti-HAV IgM in a jaundiced patient confirms the diagnosis of hepatitis A. The titers of IgM antibody decline over the next few months. IgG antibodies are slower to appear but can be detected in all patients after 3 weeks of disease. The presence of HAV IgG antibody in a patient in the absence of HAV IgM antibody denotes previous infection with HAV with full recovery.
C. The treatment of hepatitis A
is supportive. Most patients do not require hospitalization. Patients should receive good nutrition and abstain from alcohol and other hepatotoxic agents. Enteric precautions are recommended to prevent fecal-oral transport. In patients with severe cholestatic hepatitis A, steroid therapy may be beneficial. Prednisone 40 mg daily may be given for several weeks and gradually tapered.
D. Prophylaxis.
Administration of normal serum immunoglobulin has been shown to provide protection if given before exposure to the virus or during the incubation period of the disease.
1. Postexposure prophylaxis
of 0.02 mg/kg is recommended for household and sexual contacts of patients with hepatitis A within 2 weeks of exposure. Similar prophylaxis is recommended for staff and members of daycare centers and institutions providing custodial care. It is not recommended for contacts in schools, offices, factories, and hospitals unless there is a problem with hygiene.
2. Travelers
to endemic areas should receive prophylaxis, with the dose of the immunoglobulin dependent on the intended period of stay. For travel of less than 2 months’ duration, 0.02 mL/kg should be given, and 0.06 mL/kg for longer periods.
3. Vaccine.
Over the last several decades, the incidence of HAV infection has been decreasing in industrialized countries. Because infection in early childhood is becoming less common, a population of susceptible adolescents and adults, in whom hepatitis A can be more severe, is emerging. Passive immunization with serum immune globulin (IG) has a limited duration of protection of approximately 3 months. For a longer duration of protection, active immunization with a vaccine is required. Clinical trials with a killed whole virus vaccine have been successful, and the vaccine is now available. The vaccine is administered in three doses intramuscularly into the deltoid muscle at 0, 1, and 6 months. A recombinant complementary DNA vaccine for this RNA virus is also available.
A combination vaccine (Twinrit-Galaxo Smith Kline) containing 20 µg of HBsAg protein (Energix -B) and >720 ELISA units of inactivated Hep A virus (HAV VIX) provides dual protection with three injections at 0, 1, and 6 months.
IV. HEPATITIS B (HB).
There are about 2.5 billion people worldwide infected with HB virus (HBV), of these about 350 million persons are chronic carriers and 4 million new HBV infections occur yearly. The highest prevalences of HBV (about 15%) are in the Far East and Southeast Asia, Middle East, Africa, and among Alaskan natives and Pacific Islanders. HBV causes acute and chronic liver disease and liver cancer.
HBV is spread parenterally (horizontal transmission) or by intimate contact since HBV is found not only in blood, but also in semen, saliva, and other body secretions. It is believed that transmission in Asia is perinatal (vertical transmission) from mother to infant.
The risk factors for transmission include high-risk sexual activity (multiple sexual partners, homosexual activity as in men having sex with men), injection drug use, hemodialysis, living or being born in an endemic area, and working in the health care profession.
There are eight genotypes (A-H) of HBV. Genotype A is found in Europe and North America, genotype B and C in the Far East and Southeast Asia, and genotype D mainly in southern Europe, Africa, and India. The clinical significance of genotypes are enfolding (i.e., genotype A and B are more sensitive to treatment with interferon than genotypes C and D).
A. HBV is a DNA virus
belonging to a class of animal viruses called hepadna viruses. These viruses are hepatotropic, tend to cause persistent infections, and have been associated with the development of hepatocellular carcinoma. HBV is unique among human viruses in its genomic and antigenic structure and its replicative cycle.
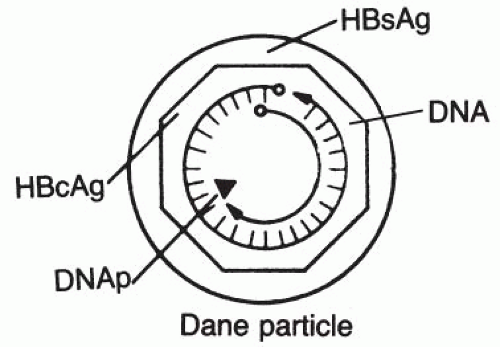
The structure of HBV is shown in
Figure 50-2. It consists of an outer shell made up of a protein (HBsAg) and a complex inner core. The complex inner core or the nucleocapsid core is a 27-nanometer (nm) icosahedral structure that consists of 180 copies of viral core protein (HBcAg) surrounding the viral DNA (genome) and virally encoded DNA polymerase. HBcAg protects the viral genome from degradation by exogenous nucleases.
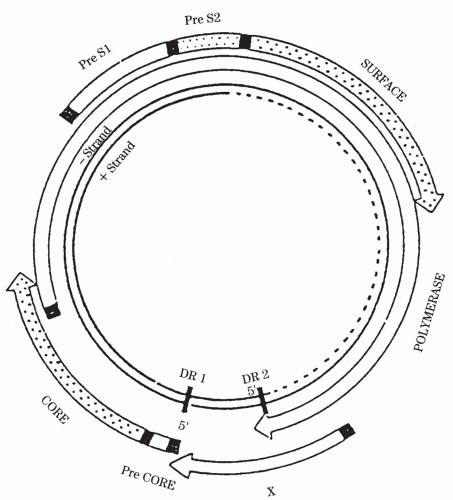
Figure 50-3 depicts the structure of the HBV genome.
The HBV genome is composed of circular DNA of approximately 3,200 base pairs with a complete negative strand and an incomplete complementary positive strand. The negative strand contains overlapping genes that encode structural proteins (surface proteins and their derivatives and core) and two replicative proteins (polymerase and X). HBV is unique among DNA viruses in that it replicates in a way similar to that of the RNA retroviruses such as the human immunodeficiency virus (HIV) via an RNA intermediate.
Once HBV is blood borne in the host, the replication cycle begins with the attachment of HBV to the hepatocyte cell membrane and entry into the hepatocyte cytoplasm. The virus is then uncoated and the nucleocapsid and the viral DNA are transported into nucleus. Once in the nucleus, the viral genome is repaired by filing in the gap in the positive-strand DNA and forming the covalently closed circular DNA (cc cDNA.) Thus, complexly double-stranded HCV DNA is formed. Synthesis of cc cDNA is catalyzed by the viral DNA polymerase.
The cc cDNA is the template for synthesis of genomic and subgenomic transcripts, catalyzed by host RNA polymerase. Genomic or pregenomic RNA thus formed then acts as the template for future DNA minus-strand synthesis.
Viral RNA transcripts are then transported into the cytoplasm and their translation into proteins yields the viral envelope, core, precore protein, and viral DNA polymerase. Viral Packaging encapsidation occurs in the cytoplasm. Encapsidation involves the assembly of the viral core consisting of 180 molecules of core proteins and the synthesis of the viral DNA by reverse transcription. The template for the minus strand is pregenomic mRNA and the template for the plus strand is minus-strand DNA. The two DNA strands of HBV are made sequentially rather than simultaneously as occurs in conventional DNA replication. Once the DNA synthesis is completed, the template RNA is degraded by specific RNAase which resides in the viral polymerase.
After replication is complete, the viral core is either transported into the nucleus or passes through the endoplasmic reticulum and Golgi apparatus where the core acquires the envelope proteins before exportation from the cell by exocytosis.
Early in HBV infection, some of the nucleocapsid cores are transported back to the nucleus where synthesis of the plus strand is completed and stable cc cDNA molecules are formed. The cc cDNA molecules form a reservoir of transcriptional templates so that after cell division of the infected hepatocytes, infection will be propagated to daughter hepatocytes. When the HBV infection is well established, nucleocapsid cores are preferentially exported from the hepatocytes facilitating the horizontal spread of infection throughout the liver.
Integration of HBV-DNA either intact or more frequently in fragments into the host genome does occur in chronic HBV infection and is implicated in hepatic carcinogenesis.
B. Pathogenesis.
At the entry of HBV and its invasion of hepatocytes, viral proteins are expressed on the hepatocyte membrane. These proteins are recognized by the host immune systems, both the humoral and the cellular arms. If the host immune response to the infected hepatocytes is strong enough to destroy all the involved cells, “hepatitis” results in clearance of the virus.
However, if the immune response is inadequate to completely obliterate the infected hepatocytes, an ongoing viral infection ensues with varying degrees of hepatic inflammatory response.
If the HBV infection occurs perinatally (i.e., by vertical transmission, before the immune system of the infant is fully developed), an inadequate immune response is mounted and viral persistence results (immune tolerance).
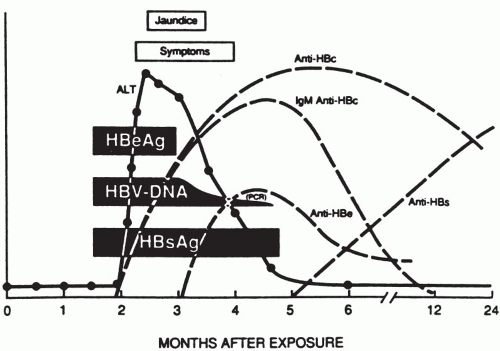
C. Serology.
Infection with HBV results in an overproduction of HBsAg outnumbering the intact virus by 10 million to 1. Assays to detect various HBV-related antigens and antibodies in the serum of infected individuals are summarized in
Figure 50-4.
Hepatitis B e antigen (HBeAg) is an internal antigen of HBcAg particles that can be detected in the serum of patients with high levels of circulating HBV. HBeAg is found only in HBsAg-positive serum and signals active ongoing infection and infectivity. HBcAg is found only within the infected hepatocytes and not in the serum. The hepatitis B antibodies (Ab) are described in the following sections.
D. HBV infection.
Infection with HBV may present in one of six clinical states: acute hepatitis, immune tolerant state, chronic hepatitis, inactive asymptomatic carrier state, and cirrhosis and hepatocellular carcinoma. Infection with HBV in older children and adults can lead to several outcomes.
Approximately two thirds of the individuals infected with HBV have a transient subclinical infection, followed by rapid clearance of the virus with a strong immune response, production of high titers of HBsAb, and permanent immunity. HBcAb is also produced in these individuals but does not confer or suggest immunity.
E. Acute hepatitis.
The outcomes of HBV infection are outlined in
Figure 50-5. About one fourth of individuals with HBV infection develop clinically apparent acute hepatitis. The incubation period, or time between exposure and onset of symptoms, is 1 to 6 months. During this time, there is active viral replication, and the patient’s serum becomes positive for HBsAg, DNA, and HBcAb IgM.
1. Clinical presentation.
Before jaundice and typical clinical findings of hepatitis become apparent, these patients may present with rash, neuralgia, arthralgia, arthritis, glomerulonephritis, and polyarteritis nodosa, vasculitis, mixed cryoglobulinemia, pericarditis, pancreatitis, and aplastic anemia. These disease states are believed to result from circulating antigen-antibody complexes.