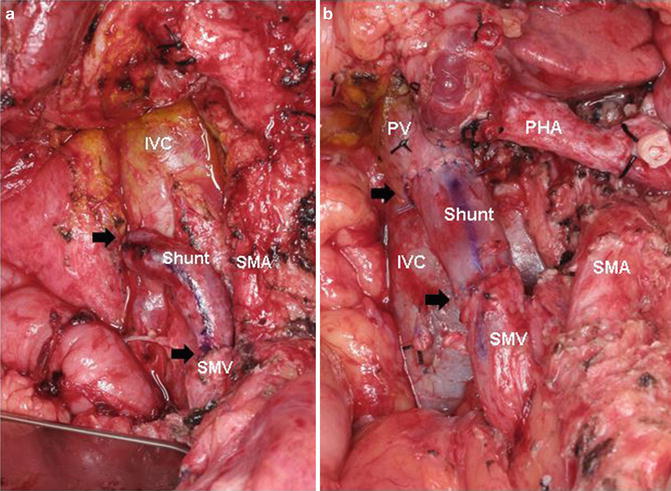
Fig. 15.1
(a, b) Coronal CT images of anatomic variations of the IMV insertion site. In Fig. 15.1a, the IMV drains into the SMV (Arrow) whereas in Fig. 15.1b, the IMV drains into the SV (Arrow). In the first scenario, if SV ligation is required to remove the SMV–PV confluence due to tumor abutment/encasement at the time of pancreaticoduodenectomy, there is no avenue for retrograde decompression of the SV other than through collaterals. This may result in sinistral portal hypertension. Alternatively, as seen in Fig. 15.1b, if the SV is ligated at the PV–SMV–SV confluence, the IMV may provide retrograde decompression of the SV. SMV–IB superior mesenteric vein’s iliac branch, IMV inferior mesenteric vein, SMV superior mesenteric vein, PV portal vein, SV splenic vein
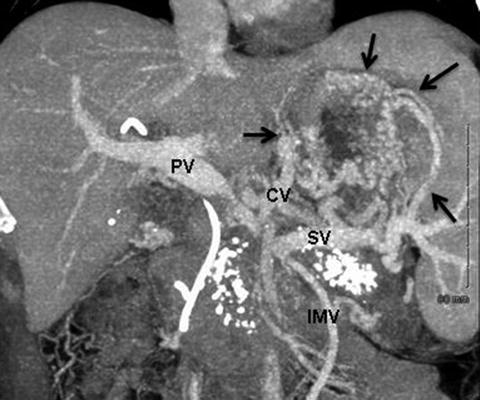
Fig. 15.2
Coronal CT venous reformatted images illustrating development of sinistral portal hypertension in a patient with chronic pancreatitis. In this patient, the PV–SMV is focally occluded and gastric varices have developed. Ligation of the SV at the confluence will only worsen this situation as the IMV will also be sacrificed. Arrows point to extensive left-sided portal hypertension. Coronary vein (CV) is significantly enlarged. PV portal vein, SV splenic vein, IMV inferior mesenteric vein
2.
Artery first approach
If the segment of SMV to be resected is distal to the SV–PV junction, the SV does not need to be divided. However, preservation of the SV (a) prevents easy access to the proximal SMA anteriorly and (b) usually necessitates interposition grafting, as the PV remains tethered at the PV–SMV–SV confluence thereby preventing the PV from having enough length to complete a primary anastomosis to the SMV. For these two reasons, some surgeons routinely divide the SV when performing segmental venous resection and reconstruction. Preservation of the SV–PV junction is important because it essentially eliminates the risk of PV thrombosis or stenosis. Alternatively, when resecting the SMV distal to the splenic-portal junction, the tumor can be separated from the SMA first, as initially described by Leach and colleagues [13]. This approach is technically challenging and is appropriate only for modest-sized tumors with short-segment SMV encasement (Fig. 15.3).
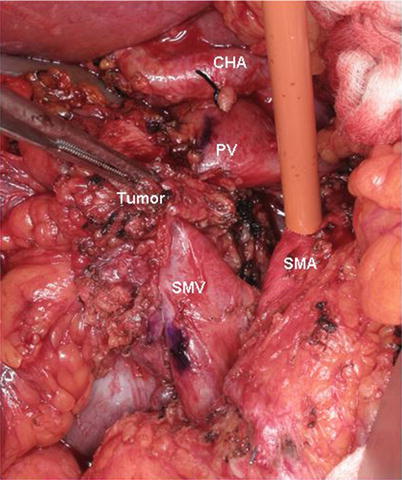

Fig. 15.3
Intraoperative photograph of a SMA first approach. A modest-sized tumor with short-segment SMV encasement is separated from the SMA by an artery-first dissection leaving the tumor/specimen attached only to the PV–SMV confluence. A Rommel tourniquet is seen encircling the SMA to provide inflow occlusion during venous resection and reconstruction thereby decreasing bowel wall edema. PV portal vein, SMV superior mesenteric vein, CHA common hepatic artery, SMA superior mesenteric artery
Venous Shunting Procedures
Mesocaval Shunts
An additional technique that can be u sed when SMV resection is required includes the creation of a mesocaval shunt. Read and colleagues first described the use of homologous vein graft as a conduit for the mesocaval shunt in 1970 [14]. Drapanas popularized the procedure for the treatment of portal hypertension in 1972 by showing efficacy in 25 patients with acute exsanguinating variceal hemorrhage. Throughout the 1970s, several authors reported success with autologous, homologous, heterologous, and synthetic mesocaval shunts for the treatment of portal hypertension and bleeding gastroesophageal varices [15]. In addition, in 2002, Orloff and coworkers published a series of 200 patients with extrahepatic portal hypertension secondary to portal vein thrombosis, who were successfully treated with portosystemic shunts. All of these patients had normal liver biopsies with normal liver function, and the majority of patients underwent mesocaval shunts with no immediate postoperative mortality [16].
We first used temporary mesocaval shunting during PD in patients with radiographic evidence of cavernous transformation of the PV due to short-segment SMV–PV occlusion. When the pancreatic head is removed in the setting of PV/SMV occlusion, collateral flow is eliminated and therefore, there is no venous outflow for the midgut. In such cases, the portal dissection should not be attempted until portal flow is diverted due to the risk for life-threatening hemorrhage if these collaterals (especially in the porta hepatis) are inadvertently entered. In the setting of cavernous transformation of the PV, PD with venous reconstruction requires an appropriate length of the SMV below (usually the main concern) and PV above the region of tumor encasement.
In addition to reducing blood loss during the dissection, temporary mesocaval shunting greatly enhances the exposure of the entire root of mesentery. Therefore, we have expanded the indications for mesocaval shunting to include patients who not only require a difficult SMV resection/reconstruction, but also those who pose a challenging SMA dissection. For example, when the tumor encases the SMV and abuts the SMA (often the posterior wall of the SMA and usually accompanied by tumor extension into the root of the small bowel mesentery), optimal SMA exposure is an absolute necessity. In such situations, we have divided the SMV and used an internal jugular vein (IJV) as an autologous interposition graft to create a temporary mesocaval shunt from the SMV to the inferior vena cava (IVC). In contrast to the previously discussed technical options for SMA exposure (medial to lateral, and artery first), this technique allows for wide exposure of the root of the mesentery and prevents any form of traction injury on the SMV–PV confluence during the SMA dissection (Fig. 15.4). These operations represent the highest level of technical complexity and are only offered to very carefully selected patients, and always after a period of induction therapy when performed for pancreatic adenocarcinoma.
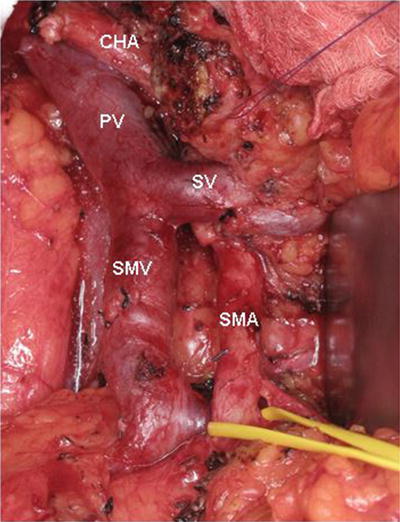

Fig. 15.4
Intraoperative photograph of a mesenteric root dissection. This maneuver provides wide exposure of the SMA and prevents any form of traction injury on the SMV–PV confluence during SMA dissection. In this image, the SMV and SMA are completely skeletonized and the SMA is encircled by a vessel loop. PV portal vein, CHA common hepatic artery, SV splenic vein, SMV superior mesenteric vein, SMA superior mesenteric artery
Surgical Technique for Mesocaval Shunt
The initial steps of PD are performed as previously described by Evans and colleagues [11, 17]. CT evidence of tumor involvement of the SMV or its first-order branches should prompt thorough scrutiny of the venous anatomy to determine the extent of vascular involvement and to develop an optimal strategy for vascular resection and reconstruction. High-quality preoperative CT imaging should prevent unexpected venous resections at the time of PD.
The SMV caudal to the area of tumor encasement should be exposed and this part of the operation is extremely difficult when tumor extends into the transverse mesocolon. The SMA dissection is critical and exposure of the SMA medial to the SMV early in the operation is strongly encouraged. Extended kocherization of the duodenum to the left lateral border of the aorta exposes the anterior surface of the left renal vein (and the location of the SMA origin can usually be appreciated at this time). An autologous left internal jugular vein (IJV) conduit is harvested to create the mesocaval shunt. The IJV graft should be marked along its anterior surface, prior to harvest, to prevent a twist during creation of the anastomoses. The IJV graft-caval anastomosis is performed first to minimize arterial inflow-occlusion time. When the first anastomosis is complete (Fig. 15.5a), we usually deliver a modest dose (2000 IU) of systemic heparin as the SMA will be temporarily occluded with a Rommel tourniquet. This technique allows arterial inflow occlusion to be limited to less than 15 min while the second anastomosis (SMV–IJV) is performed. Systemic heparinization is not mandatory and we use heparin only when the remaining dissection will not be significantly complicated by the use of anticoagulation. We divide the SMV just cephalad to its bifurcation into the jejunal and ileal branches. Whenever possible we try to preserve the jejunal branch, but if necessary, it can be divided. The IJV–SMV anastomosis completes the mesocaval shunt, connecting the SMV cephalad to its jejunal-ileal bifurcation to the IJV which is connected to the IVC (Fig. 15.5a). The anastomoses are completed end-to-end with interrupted 6-0 prolene sutures. Creation of the SMV–IJ–IVC mesocaval shunt will completely expose the root of the small bowel mesentery and provides excellent SMA exposure. Once all portal flow is diverted, the portal dissection can then be safely completed and the PV at the superior border of the pancreas is divided with an endovascular GIA stapler. The specimen is then removed and sent to pathology. The IVC–IJV end of the mesocaval shunt is then detached, cut to the appropriate length, and anastomosed to the PV forming the final interposition graft anastomosis (Fig. 15.5b).